1. Background
Chronic anemia is common in patients with end-stage chronic kidney disease treated with maintenance hemodialysis (MHD). It is associated with decreased quality of life, increased cardiovascular events, and mortality in this patient group. Factors such as erythropoietin, amino acids, iron, and folic acid are associated with reduced red blood cell synthesis in chronic kidney disease (CKD) patients with and without dialysis (1-3). Iron is an essential component in hematopoiesis, and iron deficiency reduces erythropoiesis in CKD patients. Assessment of iron stores in anemic MHD patients is essential for diagnosing and treating anemia. Ferritin concentrations and transferrin saturation (TSAT) should be used to assess iron status. Based on these two indicators, it is possible to determine whether the patient has iron deficiency or iron overload. Iron overload is indicated by increased plasma ferritin levels. Additionally, high ferritin levels can show that the patient has an inflammatory reaction, which is common in MHD patients with anemia, although it is not contagious (4, 5). C-reactive protein (CRP) is a biomarker that indicates acute infection. In MHD patients, CRP is also considered a cardiovascular risk factor (6-8). Iron overload in MHD patients is related to many factors, including inflammation. Ruiz-Jaramillo Mde et al. demonstrated an association between iron overload and several inflammatory markers, including CRP and Interleukin 6 (IL-6) (9). Ishida and Johansen also discussed the association between iron overload and infection risk (10). From the above premises, we hypothesized that elevated plasma CRP is related to iron overload in anemic MHD patients.
2. Objectives
We performed this study to determine the ratio of iron overload and the relationship with elevated plasma C-reactive protein (CRP) concentration in maintenance hemodialysis (MHD) patients with anemia.
3. Methods
3.1. Patients
We conducted a cross-sectional study on all MHD patients (n = 168) treated at the Department of Nephrology and Hemodialysis, Military Hospital 103, Ha Noi, Vietnam. Data were collected from February 2022 to April 2023. We excluded patients who were under 18 years old, suspected of having a surgical disease, showing signs of infection, had a recent blood transfusion in the last 4 weeks, had received intravenous iron in the previous 14 days, suffered acute blood loss within the last 3 months, experienced bleeding during the study period, or were pregnant or breastfeeding. Additionally, patients who did not agree to participate in the study were excluded. The remaining 103 patients provided written informed consent before participation in our study.
We collected all data on clinical characteristics and laboratory parameters at the baseline of the study. Blood was drawn just before the start of a dialysis session in a non-fasting state to measure serum albumin, creatinine, urea, hemoglobin, and hematocrit. Serum β2-microglobulin and parathyroid hormone (PTH) concentrations were measured using the latex immunoassay principle at enrollment. C-reactive protein was estimated quantitatively using a solid-phase ultrasensitive enzyme immunoassay.
The diagnosis of anemia was based on hemoglobin levels of < 130 g/L (13 g/dL) for men and < 120 g/L (12 g/dL) for women, according to the KDIGO 2012 recommendations. The primary renal disease clinical diagnoses included chronic glomerulonephritis, hypertensive nephropathy, chronic pyelonephritis, diabetic nephropathy, gout, and lupus nephropathy.
Plasma iron and ferritin concentrations were also quantified. Total iron-binding capacity (TIBC) was estimated using the ELISA method. Transferrin saturation (TSAT) was calculated using the formula: TSAT (%) = (plasma iron × 100)/plasma TIBC. Iron overload was defined as a ferritin concentration > 800 ng/mL or a TSAT > 50%, as recommended by the United Kingdom Renal Association 2017 (11).
3.2. Statistical Analysis
The continuous data were summarized using mean and standard deviation and analyzed using ANOVA and Student's t-test. Categorical data were presented using frequency and percentage and analyzed using the chi-square test. Multivariate regression analysis was conducted to determine independent factors, and receiver operating characteristic (ROC) curves were used to predict iron overload, with the area under the curve (AUC) calculated. We used the Statistical Package for Social Sciences (SPSS) version 20.0 (Chicago, IL, USA) for statistical analysis. A significance level of P < 0.05 was considered statistically significant.
4. Results
Table 1 shows that the median age of the study group was 54 years. There was an equal proportion of men and women. The proportions of diabetes mellitus and underweight patients were 14.6% and 10.7%, respectively. The MHD patients had low mean hemoglobin and CRP concentrations and high plasma Beta2-microglobulin and PTH concentrations. Up to 59.2% of patients had lipid disorders, and 30.1% had hepatitis infections.
| Clinical Characteristics and Laboratory Parameters | Mean ± SD/Median | No. (%) |
|---|---|---|
| Age (y) | 54 (43 - 66) | N/A |
| Gender | ||
| Males | N/A | 56 (54.4) |
| Females | N/A | 47 (45.6) |
| Etiology | ||
| CGN | N/A | 60 (58.3) |
| CPN | N/A | 11 (10.7) |
| Gout | N/A | 2 (1.9) |
| Lupus | N/A | 2 (1.9) |
| Hypertension | N/A | 13 (12.6) |
| DM | N/A | 15 (14.6) |
| BMI (kg/m2) | ||
| < 18.5 | N/A | 11 (10.7) |
| 18.5 - 22.9 | N/A | 73 (70.9) |
| ≥ 23 | N/A | 19 (18.4) |
| Average | 20.84 ± 2.31 | N/A |
| Hemodialysis duration (mo) | 36 (12 - 60) | N/A |
| Urea (mmol/L) | 26.81 (21.38 - 31.95) | N/A |
| Creatinine (µmol/L) | 898.92 (731.19 - 1079.39) | N/A |
| Albumin (g/L) | 38.25 ± 3.25 | N/A |
| CRP (mg/L) | 2.16 (1.06 - 5.32) | N/A |
| Lipid disorder | N/A | 61 (59.2) |
| Hemoglobin (g/L) | 87.58 ± 18.36 | N/A |
| MCV (fL) | 89.95 ± 7.26 | N/A |
| MCH (pg) | 29.53 ± 2.89 | N/A |
| MCHC (g/L) | 328.16 ± 13.37 | N/A |
| Beta 2-Microglobulin (mg/L) | 30.6 (29.4 - 31.53) | N/A |
| PTH (pg/mL) | 408.6 (151.3 - 970.4) | N/A |
| Hepatitis B or C Infection | N/A | 30 (30.1) |
Abbreviations: CGN, chronic glomerulonephritis; CPN, Chronic Pyelonephritis; DM, diabetic mellitus; BMI, Body Mass Index; CRP, C-reactive protein; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobine; MCHC, mean corpuscular hemoglobine concentration; PTH, parathyroid hormone. N/A, not applicable.
The results in Table 2 show that MHD patients had a high proportion of iron overload (up to 51.5%) and iron deficiency (43.7%). Only 4.9% of patients had normal iron status. The median values of ferritin and TSAT were 239.4 ng/mL and 27.26%, respectively.
| Clinical Characteristics and Laboratory Parameters | Mean ± SD/ Median | No. (%) |
|---|---|---|
| Serum iron (µmol/L) | 10.28 (8.08 - 13.74) | N/A |
| Serum ferritin (ng/mL) | 239.4 (105.1 - 502.3) | N/A |
| Serum TIBC (µmol/L) | 49.86 (19.0 - 79.42) | N/A |
| TSAT (%) | 27.26 (11.87 - 44.38) | N/A |
| Iron status | ||
| Iron deficiency | N/A | 45 (43.7) |
| Normal iron | N/A | 5 (4.9) |
| Iron overload | N/A | 53 (51.5) |
Abbreviations: TIBC, total iron-binding capacity; TSAT, Transferrin saturation. N/A, not applicable.
Table 3 shows almost no difference in clinical characteristics and laboratory parameters between the two groups of patients with and without iron overload. However, the plasma CRP concentration in patients with iron overload was significantly higher than in those without iron overload (P < 0.001).
| Clinical Characteristics and Laboratory Parameters | Iron Overload (n = 53) | Without Iron Overload (n = 50) | P-Value |
|---|---|---|---|
| Ages (y) | 56 (44.5 - 65.5) | 50.5 (40 - 67) | 0.444 |
| Gender | 0.942 | ||
| Males | 29 (54.7) | 27 (54) | |
| Females | 24 (45.3) | 23 (36) | |
| Etiology | 0.762 | ||
| CGN | 32 (60.4) | 28 (56) | |
| CPN | 4 (7.5) | 7 (14) | |
| Gout | 2 (3.8) | 0 (0) | |
| Lupus | 1 (1.9) | 1 (2) | |
| Hypertension | 7 (13.2) | 6 (12) | |
| DM | 7 (13.2) | 8 (16) | |
| BMI (kg/m2) | 0.515 | ||
| < 18.5 | 7 (13.2) | 4 (8) | |
| 18.5 - 22.9 | 38 (71.7) | 35 (70) | |
| ≥ 23 | 8 (15.1) | 11 (22) | |
| Average | 20.61 ± 2.19 | 21.09 ± 2.42 | 0.296 |
| Hemodialysis duration (Mo) | 31 (15.5 - 66) | 36 (12 - 63) | 0.866 |
| Urea (mmol/L) | 26.81 (21.79 - 32.33) | 27.02 (21.0 - 31.26) | 0.712 |
| Creatinine (µmol/L) | 866.92 (754.98 - 1078.36) | 902.29 (711.32 - 1086.04) | 0.744 |
| Albumin (g/L) | 37.90 ± 3.25 | 38.62 ± 3.25 | 0.264 |
| CRP (mg/L) | 3.15 (1.66 - 6.67) | 1.5 (0.78 - 3.24) | < 0.001 |
| Lipid disorder | 34 (64.2) | 27 (54) | 0.295 |
| Hemoglobin (g/L) | 85.81 ± 18.87 | 89.46 ± 17.8 | 0.316 |
| Anemia | 51 (96.2) | 49 (98) | 1.000 |
| MCV (fL) | 90.8 ± 7.41 | 89.06 ± 7.07 | 0.225 |
| MCH (pg) | 29.82 ± 3.01 | 29.22 ± 2.75 | 0.295 |
| MCHC (g/L) | 328.15 ± 13.61 | 328.18 ± 13.26 | 0.991 |
| Beta 2-Microglobulin (mg/L) | 30.7 (29.35 - 31.51) | 30.59 (29.6 - 31.64) | 0.935 |
| PTH (pg/mL) | 472.7 (213.47 - 1138.67) | 353.7 (138.3 - 970.4) | 0.197 |
| Hepatitis B or C Infection | 18 (33.96) | 13 (26.0) | 0.379 |
Abbreviations: CGN, chronic glomerulonephritis; CPN, chronic pyelonephritis; DM, diabetic mellitus; BMI, Body Mass Index; CRP, C-reactive protein; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobine; MCHC, mean corpuscular hemoglobine concentration; PTH, parathyroid hormone.
a Values are expressed as No. (%) or Mean ± SD.
The multivariate logistic regression analysis results in Table 4 show that plasma CRP was an independent variable associated with iron overload in MHD patients.
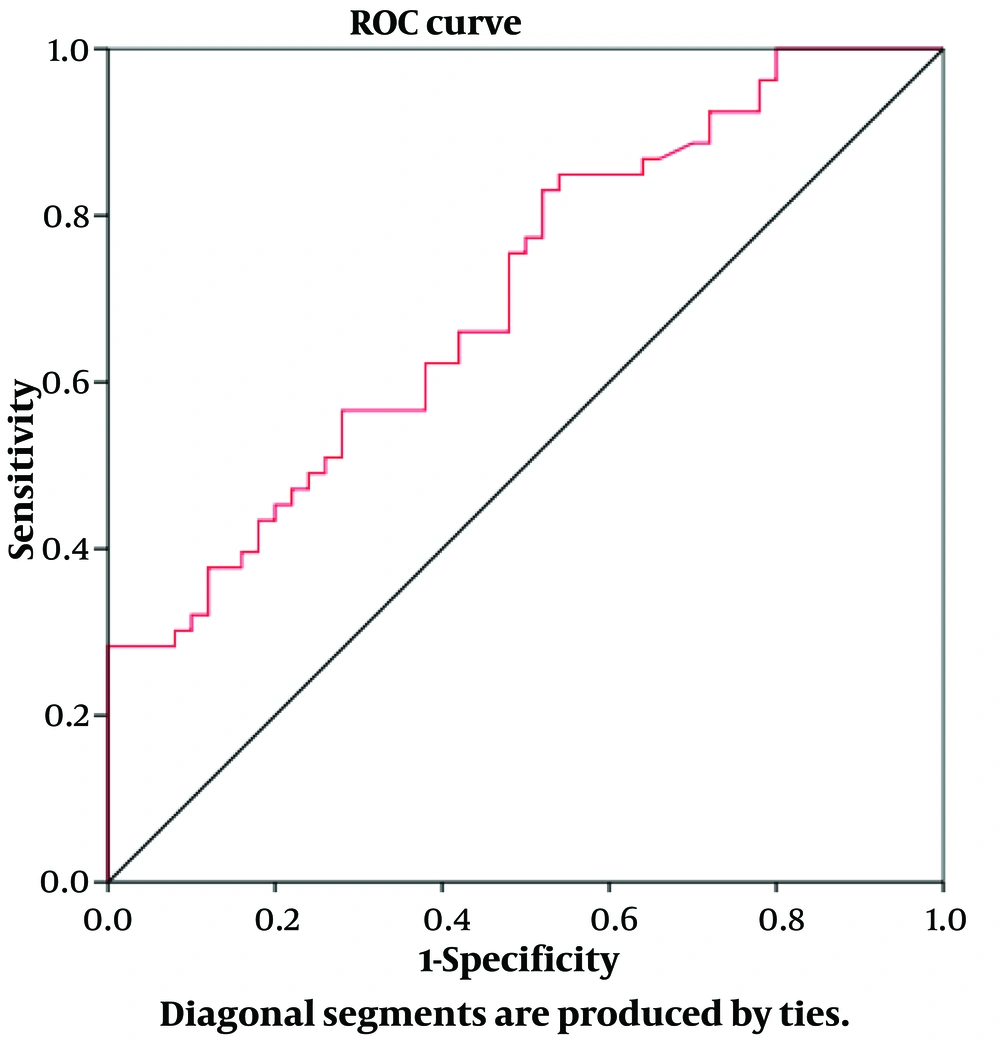
The ROC curve model in Figure 1 shows that CRP was a valuable predictor of iron overload in MHD patients.
| Variables | OR | 95% Cl | P-Value |
|---|---|---|---|
| Age | 0.995 | 0.963 - 1.027 | 0.746 |
| Hemodialysis duration | 0.998 | 0.99 - 1.027 | 0.584 |
| BMI | 0.887 | 0.727 - 1.006 | 0.24 |
| Hemoglobin | 0.989 | 0.966 - 1.014 | 0.386 |
| MCV | 1.042 | 0.879 - 1.236 | 0.636 |
| MCH | 1.011 | 0.662 - 1.545 | 0.959 |
| CRP | 1.351 | 1.106 - 1.65 | 0.003 |
| Hepatitis B or C Infection | 1.464 | 0.626 - 3.425 | 0.379 |
Abbreviations: BMI, Body Mass Index; MCV, Mean Corpuscular Volume; MCH, Mean Corpuscular Hemoglobin; CRP, C-reactive protein.
5. Discussion
5.1. Iron Overload in MHD Patients
Anemia is common in MHD patients (12, 13). In this study, we selected only anemic patients to evaluate iron overload in dialysis patients with anemia and identify factors associated with iron overload. Our results show that the proportion of iron overload was 51.5%. Iron overload is a significant concern, as reported by many studies worldwide, with high rates in MHD patients. Each study showed different iron overload rates due to varying evaluation criteria. Many indicators can be used to diagnose clinical iron overload, including plasma ferritin concentration and/or TSAT, and measuring cardiac and hepatic iron.
Canavese et al. used a superconducting quantum interference device (SQUID) to make direct noninvasive magnetic measurements of nonheme hepatic iron content in 40 dialysis patients. The results showed that only 12 out of 40 (30%) patients had normal hepatic iron content (SQUID < 400 µg/g), indicating that 70% of patients had iron overload (14). Rostoker et al. measured liver iron concentration using T1 and T2* contrast magnetic resonance imaging (MRI) without gadolinium in 119 HD patients. The research results showed that the rate of iron overload in the liver was up to 84% (100/119 patients), with up to 36% of patients having severe overload with liver iron concentration > 201 μmol/g of dry weight (15). Using T2*MRI to evaluate iron overload in 30 MHD patients, Delibaş et al. found that 76.6% (23/30 patients) had iron overload (16).
Iron overload occurs in many different diseases, increasing total body iron stores and leading to target organ damage. Iron replacement therapy for anemia in CKD patients can result in secondary iron overload (hemochromatosis) (17). The sequelae of iron overload often appear in iron storage organs: It can lead to cirrhosis in the liver, cause arrhythmias and myocardial damage in the heart, and eventually result in heart failure (18-20). When iron accumulates in the thyroid, pancreas, and pituitary gland, it can cause dysfunction of these organs (21). Treating iron overload in CKD patients is complicated because these patients often have reduced glomerular filtration rates, making it challenging to eliminate iron chelators (22). Therefore, it is necessary to balance the correction of iron deficiency anemia while preventing iron overload in clinical practice (23, 24).
5.2. Plasma CRP Predicts Iron Overload
To determine the factors related to iron overload in MHD patients, we compared the clinical and subclinical parameters between the two groups: Those with iron overload and those without. In this study, we used CRP rather than hs-CRP because CRP quantification is more accessible and cost-effective in many medical facilities. The results showed that plasma CRP was associated with iron overload. The plasma CRP concentration in the iron-overloaded group was significantly higher than in the non-iron-overloaded group (P < 0.001) (Table 3). We also identified plasma CRP as an independent factor associated with iron overload (P = 0.003) (Table 4). Notably, plasma CRP was a good predictor for iron overload, with an AUC of 0.703 (P < 0.001).
Maintenance hemodialysis patients are at risk of iron deficiency due to blood loss during dialysis and reduced absorption caused by gastrointestinal disorders. Therefore, iron supplementation using recombinant erythropoiesis-stimulating agents (ESA) is a strategy for treating anemia. However, iatrogenic iron overload is a concern in this group of patients. Our study shows that up to 51.5% of patients have iron overload; other studies have shown rates of 70% (12) and 84% (15). Research by Canavese et al. also analyzed the association between plasma CRP and iron overload. These authors found that the plasma CRP concentration in the mild iron overload group (n = 13 patients) was higher than in the non-iron overload group (n = 12 patients), but the difference was not statistically significant (P = 0.28) (14). However, Ruiz-Jaramillo Mde et al. studied 143 children and adolescents, including 48 on peritoneal dialysis (PD), 53 on MHD, and 42 post-renal transplantation. They found 15 children and adolescents with iron overload; 11 were in the MHD group, and 4 were in the PD group. The plasma IL-6 and CRP concentrations in the iron overload group were higher than in those without iron overload (P = 0.04 and P = 0.01, respectively) (9).
In a cross-sectional study, Rostoker et al. also showed a positive correlation between liver iron concentration and plasma CRP concentration (P < 0.05) (15). CRP is a biomarker of acute inflammatory response. The positive relationship between elevated plasma CRP and iron overload has a pathogenic mechanism. An association between iron overload and infectious diseases has been documented, with iron overload increasing the risk of bacterial and viral growth (25). Chronic inflammatory conditions such as DM are associated with increased iron stores and overload (26). Thus, based on plasma CRP, we can predict iron overload, which is helpful for clinicians who need to assess iron stores when there is an increase in plasma CRP concentration.
This study has the following limitations: It is a single-center study, so the sample size is not large, and the age range is not evenly distributed across all ages. Additionally, because iron overload is only determined based on ferritin and TSAT, the manifestations of iron overload in the patient's liver, heart, and other organs were not observed.
5.3. Conclusions
The proportion of iron overload was 51.5%. Plasma CRP was a good predictor of iron overload in maintenance hemodialysis patients with anemia.