1. Background
Percutaneous nephrolithotomy (PCNL) is the standard treatment for large renal stones, but it is associated with potential complications such as urinary tract infection (UTI), fever, and sepsis. Recent studies report incidence rates of 27.6% for UTI, 9.5% for fever, and 4.5% for sepsis following PCNL (1). To mitigate these complications, prophylactic antibiotic use is recommended by guidelines such as those from the European Association of Urology (EAU) (2) and the American Urological Association (AUA) (3). Some studies have also suggested that extended courses of prophylactic antibiotics may be more effective than a single dose in reducing infection-related complications, particularly in high-risk patients (4).
The AUA guidelines recommend several antibiotics for PCNL prophylaxis, including first- and second-generation cephalosporins, aminoglycosides combined with metronidazole or clindamycin, ampicillin/sulbactam, or a fluoroquinolone (5). A single dose of prophylactic antibiotic is also recommended during the removal of the nephrostomy tube (5).
When comparing cefazolin and ceftizoxime for prophylaxis in PCNL surgery, several factors should be considered, such as spectrum of activity, pharmacokinetics, effectiveness, and safety profile. Cefazolin, a first-generation cephalosporin, is effective against a broad range of bacteria, including Staphylococcus aureus and Escherichia coli. It has a half-life of approximately 1.8 hours, allowing for rapid absorption and excretion, which results in high tissue concentrations beneficial for surgical prophylaxis. Cefazolin has been widely recognized for its effectiveness in reducing postoperative infections in various surgical procedures, including urological surgeries. Its safety profile is generally well-tolerated, although allergic reactions may occur, particularly in patients with a history of penicillin allergy (6).
Ceftizoxime, a third-generation cephalosporin, has a broader spectrum of activity compared to cefazolin, particularly against resistant strains of gram-negative bacteria, such as certain Enterobacteriaceae. Its pharmacokinetics are comparable to cefazolin, but it has a longer half-life (approximately 2 hours), allowing for less frequent dosing. Ceftizoxime is highly effective in preventing infections, especially in cases where there is a higher risk of gram-negative organisms. However, it is not as commonly used in urological surgeries as cefazolin. Ceftizoxime's safety profile is generally considered safe, though it may cause more adverse effects than cefazolin. Additionally, the broader range of action with ceftizoxime increases the risk of clostridium difficile infection (7).
Cefazolin is typically the preferred choice for infection prevention during PCNL procedures due to its effectiveness against the common pathogens involved in urological infections, especially in clean or clean-contaminated surgeries. Ceftizoxime may be a better option for cases involving resistant gram-negative organisms due to its wider coverage, but this broad-spectrum action can also negatively impact normal flora (7).
In PCNL, cefazolin is favored because of its proven efficacy against typical urological pathogens, especially in non-complicated cases. In contrast, ceftizoxime may be considered when resistant gram-negative organisms are a concern, though its wider spectrum may lead to complications such as alterations in normal flora. In terms of administration, cefazolin is generally given as a one-time dose before surgery, while ceftizoxime may require multiple doses due to its pharmacokinetics. Cefazolin is also more cost-effective and readily available compared to ceftizoxime, making it the more practical choice for routine use.
Overall, cefazolin remains the top choice for antibiotic prophylaxis in PCNL due to its established safety, efficacy, and quick response against common pathogens. Ceftizoxime may be reserved for special cases involving a higher risk of resistant infections, but it is not commonly used for this purpose. The choice of antibiotic should consider patient-specific risk factors, local resistance patterns, and hospital protocols (6, 7).
The World Health Organization (WHO) highlighted the global challenge of increasing bacterial resistance to cephalosporins and fluoroquinolones in its 2014 global monitoring report on antibiotic resistance. Given the rise in resistance and the associated complications, such as fever, sepsis, and UTIs, selecting appropriate antibiotics is critical to minimizing complications and maximizing effectiveness (8). In 2008, the National Hospital Evaluation Program (NHEP) implemented comprehensive measures for managing antibiotic resistance, which included evaluating prophylactic antibiotics used in surgeries. This program discouraged the overuse of third-generation cephalosporins, aminoglycosides, combinations of β-lactams with aminoglycosides, and vancomycin combinations (9).
It is essential to carefully assess the potential risks and benefits of combining cephalosporins with aminoglycosides for prophylaxis in patients undergoing PCNL, based on current literature and guidelines. The benefits may include the broad-spectrum protection provided by cephalosporins and aminoglycosides. Cephalosporins are effective against a wide range of Gram-positive and Gram-negative bacteria, making them suitable for infection prevention in surgical settings. Aminoglycosides, on the other hand, are particularly potent against Gram-negative bacteria, such as Pseudomonas aeruginosa, a common cause of urinary tract infections. The concurrent use of cephalosporins and aminoglycosides may also have a synergistic effect, enhancing bacterial eradication, especially in polymicrobial infections.
Research has shown that using appropriate antibiotic prophylaxis can significantly reduce the incidence of surgical site infections (SSIs), leading to better postoperative outcomes and shorter hospital stays. By covering a wide range of potential pathogens, this combination could also help prevent UTIs, which are frequent complications following urinary surgeries.
Nevertheless, the combination of cephalosporins and aminoglycosides for prophylaxis in patients undergoing PCNL comes with multiple potential risks. Overuse of broad-spectrum antibiotics, such as third-generation cephalosporins and aminoglycosides, can contribute to the emergence of antibiotic-resistant bacteria—a significant concern emphasized by the NHEP. Additionally, aminoglycosides are known for their nephrotoxic potential, especially in patients with pre-existing kidney conditions or those receiving other nephrotoxic medications. This risk is particularly critical in a population already undergoing kidney-related surgery. Furthermore, both classes of antibiotics carry the potential for allergic reactions, gastrointestinal disturbances, and other side effects. The risk of adverse effects increases when multiple antibiotics are used simultaneously.
Broad-spectrum antibiotics can also disrupt the balance of normal flora, leading to opportunistic infections such as clostridioides difficile colitis. The use of multiple antibiotics can complicate treatment regimens, increasing both the cost and the risk of medication errors.
The optimal choice of prophylactic antibiotics prior to PCNL surgery remains a subject of debate among surgeons. To address this uncertainty, we conducted a comparative study between first-generation cephalosporin (cefazolin) and third-generation cephalosporin (ceftizoxime).
2. Objectives
The objective of our study was to investigate the use of these prophylactic antibiotics and their impact on general complications, hospitalization duration, and the time to return to normal life, by analyzing the relationship between antibiotic use and complication rates.
3. Methods
3.1. Population, Sampling and Inclusion and Exclusion Criteria of Study
The study population for this research consisted of patients who underwent PCNL and were confirmed to have urinary tract stones through ultrasound or CT scan. These patients were referred to Razi, Golsar, and Pars hospitals in Rasht city and underwent the procedure in the complete supine position between 2013 and 2022.
Sampling was conducted using the availability sampling technique, selecting patients who underwent PCNL in a fully supine position at the aforementioned hospitals between 2013 and 2022. All eligible patients from these hospitals were included in the study.
The inclusion criteria for this study involved patients diagnosed with urinary stones, as confirmed by imaging methods such as CT scan and/or ultrasound, who met the criteria for PCNL. Key factors for PCNL eligibility included the size of the stones (typically greater than 2 cm), their location (e.g., within the kidney or upper ureter), and related symptoms such as severe pain, obstruction, or recurrent UTIs. Additionally, patients had to be suitable for undergoing surgery in the complete supine position, a requirement crucial to the surgical procedure. Furthermore, patients were required to have comprehensive medical documentation to provide a complete understanding of their medical history. Post-procedure follow-up was mandatory to monitor outcomes and identify any potential complications.
Individuals with ongoing urinary tract infections were excluded from this study. Patients experiencing current urinary tract infections that could complicate surgery or affect outcomes were not eligible. Additionally, individuals with anatomical abnormalities were excluded from the research. This refers to patients with structural issues in their urinary system, such as a blockage at the ureteropelvic junction, which could interfere with the surgery or influence the results.
Patients with recent hospitalizations were also not included in the study. Those who had been admitted to the hospital for any reason prior to the study may have had underlying health conditions that could affect surgical risk. Furthermore, patients who had previously used antibiotics were excluded, as prior antibiotic use could influence the presence of resistant bacteria and complicate infection management.
Patients with incomplete or inaccurate medical records, lack of access for follow-up, withdrawal of consent, or loss to follow-up were also excluded, as these factors would hinder a proper evaluation of their health status and the outcomes of the study.
3.2. Study Groups
In the first-generation (cefazolin) group, a total of 408 patients received 1 mg/kg of cefazolin intravenously, administered 30 minutes before the start of surgery. Cefazolin was continued every 6 hours post-surgery. The third-generation (ceftizoxime) group consisted of 103 patients who were given 1 mg/kg of ceftizoxime intravenously, 30 minutes prior to surgery. Ceftizoxime was then administered every 12 hours post-surgery until discharge.
In addition to the antibiotics, all patients in both groups also received aminoglycosides intravenously. The aminoglycoside was administered 30 minutes before the surgery and continued every eight hours post-surgery until discharge. After hospital discharge, all patients were followed up to monitor postoperative complications and assess the effectiveness of the prophylactic antibiotics on their outcomes.
3.3. Variables of Study
The variables in this study included: Age (years), gender, weight (kg), height (cm), BMI (kg/m2), glomerular filtration rate (GFR) (mL/min/1.73 m2), stone burden (mm), anesthesia time (minutes), operation time (minutes), hospitalization duration (days), postoperative analgesic dosage (mg), duration of postoperative analgesic use (days), return to normal life (days), history of antibiotic therapy, history of UTI, history of urine culture, underlying diseases (hypertension, diabetes, ischemic heart disease), history of stone operations, history of extracorporeal shock wave lithotripsy (ESWL), and presence of hydronephrosis.
To diagnose UTI in patients, urine samples were collected. For patients with urinary control, samples were obtained after cleansing the perineal area. For patients without urinary control, samples were collected either via suprapubic aspiration or catheterization. If these methods were not feasible, a urine bag sample was used. The urine sample was immediately tested and sent to the laboratory for complete analysis and culture (10, 11). Urinary tract infection diagnosis required both pyuria (increased white blood cells in the urine) and at least 50,000 colony-forming units (CFU) per milliliter of a uropathogenic organism. A strip test for leukocyte esterase and nitrite was also used to aid in diagnosis (12, 13).
Urine culture was performed by inoculating 0.1 mL of the urine sample onto blood agar and eosin methylene blue (EMB) agar, with incubation at 37°C for 24 to 48 hours. A positive urine culture was defined as more than 100,000 colonies from a pathogen collected via catheter or more than 50,000 colonies from suprapubic sampling (13, 14). Additionally, leukocyturia (presence of leukocytes in urine) and leukocytosis (elevated white blood cell count) were assessed as potential indicators of infection. Considering antibiotic resistance, empirical treatment for UTI was carefully selected, particularly when recent resistance trends were noted. Urinalysis was also performed to assess the presence of white blood cells, red blood cells, epithelial cells, bacteria, hyaline casts, and abnormal crystals in the urine sediment (15-17).
3.4. Statistical Analysis
In this study, the outcome variables, including postoperative complications and return to normal life following PCNL surgery, were compared between the cefazolin (first-generation) and ceftizoxime (third-generation) groups, both of which received aminoglycosides as prophylactic antibiotics. To compare numerical variables, t-tests were utilized, while chi-squared tests were applied for categorical variables, enabling the evaluation of differences in postoperative complications and time to return to normal life between the two groups. Furthermore, logistic regression was employed to calculate crude and adjusted odds ratios (OR) with 95% confidence intervals (CI), to determine the independent effect of prophylactic antibiotics on postoperative complications. All data analyses were conducted using the statistical software SPSS version 16 (USA).
3.5. Sample Size
Based on the study by Martinusen et al. (18), with a 5% error level and 95% power, the minimum required sample size for each group is estimated to be 70. Considering a potential dropout rate of 20%, the minimum sample size is adjusted to 84 for each group.
3.6. Ethics Statement
The present study protocol was reviewed and approved by the Ethics Committee of Guilan University of Medical Sciences, under approval number IR.GUMS.REC.1401.269. Informed consent was obtained from all patients before enrollment in the study. The study adhered to all relevant laws and regulations throughout the research period, and the hospital's research ethics review committee also provided their approval before the commencement of the study.
4. Results
In this study, a total of 511 patients were included. Of these, 408 patients were in the first-generation cephalosporin (cefazolin) group, while 103 patients were in the third-generation cephalosporin (ceftizoxime) group. There was a significant difference in the percentage of patients with a history of antibiotic therapy between the two groups. In the cefazolin group, 80.4% of patients had a history of antibiotic therapy, whereas in the ceftizoxime group, only 33.3% had such a history (P = 0.000). Similarly, a significant difference was observed in the percentage of patients with a history of UTI, with 7.1% in the cefazolin group and 50.9% in the ceftizoxime group (P = 0.000). Overall, the ceftizoxime group had a significantly higher percentage of patients with a history of UTI compared to the cefazolin group (P = 0.000).
Table 1 shows the frequency and percentage of demographic characteristics and risk factors of patients undergoing PCNL surgery with antibiotic prophylaxis, using either first-generation (cefazolin) or third-generation (ceftizoxime) cephalosporins in combination with aminoglycosides. A significant difference in the percentage of patients with underlying diseases was noted between the two groups: 45.3% in the cefazolin group and 64.8% in the ceftizoxime group (P = 0.000). The ceftizoxime group had a significantly higher percentage of patients with underlying diseases compared to the cefazolin group (P = 0.000).
| Variables | 1st Generation (Cefazolin) (n = 408) | 3rd Generation (Ceftizoxime) (n = 103) | P-Value |
|---|---|---|---|
| Gender | 0.407 | ||
| Male | 226 (55.4) | 55 (50.9) | |
| Female | 182 (44.6) | 53 (49.1) | |
| BMI group | 0.686 | ||
| < 19.99 | 12 (2.9) | 3 (2.8) | |
| 20 - 24.9 | 110 (27.0) | 23 (21.3) | |
| 25 - 29.9 | 179 (43.9) | 51 (47.2) | |
| ≥ 30 | 107 (26.2) | 31 (28.7) | |
| History of antibiotic therapy | 0.000 | ||
| No | 80 (19.6) | 72 (66.7) | |
| Yes | 328 (80.4) | 36 (33.3) | |
| History of UTI | 0.000 | ||
| No | 379 (92.9) | 53 (49.1) | |
| Yes | 29 (7.1) | 55 (50.9) | |
| History of urine culture | 0.70 | ||
| Negative | 260 (74.7) | 69 (76.7) | |
| Positive | 88 (25.3) | 21 (23.3) | |
| Underline disease | 0.000 | ||
| No | 223 (54.7) | 38 (35.2) | |
| Yes | 185 (45.3) | 70 (64.8) | |
| Hypertension | 0.000 | ||
| No | 296 (72.5) | 48 (44.4) | |
| Yes | 112 (27.5) | 60 (55.6) | |
| Diabetes | 0.014 | ||
| No | 325 (79.7) | 74 (68.5) | |
| Yes | 83 (20.3) | 34 (31.5) | |
| Ischemic heart disease | 0.000 | ||
| No | 392 (96.1) | 88 (81.5) | |
| Yes | 16 (3.9) | 20 (18.5) | |
| Stone operation history | 0.285 | ||
| No | 253 (62.0) | 73 (67.6) | |
| Yes | 155 (38.0) | 35 (32.4) | |
| History of ESWL | 0.001 | ||
| No | 236 (57.8) | 81 (75.7) | |
| Yes | 172 (42.2) | 26 (24.3) | |
| Hydronephrosis | 0.000 | ||
| No | 129 (31.6) | 10 (9.3) | |
| Yes | 278 (68.1) | 98 (90.7) |
Abbreviation: UTI, urinary tract infection.
a Values are expressed as No. (%).
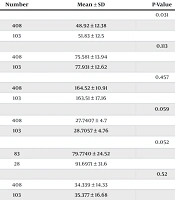
According to Table 2, the mean age of patients in the first-generation cephalosporin (cefazolin) group was 48.92 ± 12.38 years, while in the third-generation cephalosporin (ceftizoxime) group, it was 51.83 ± 12.5 years. Statistical analysis revealed a significant difference in the ages between the two groups (P = 0.031).
| Variables and Study Groups | Number | Mean ± SD | P-Value |
|---|---|---|---|
| Age (y) | 0.031 | ||
| 1st generation (cefazolin) | 408 | 48.92 ± 12.38 | |
| 3rd generation (ceftizoxime) | 103 | 51.83 ± 12.5 | |
| Weight (kg) | 0.113 | ||
| 1st generation (cefazolin) | 408 | 75.581 ± 13.94 | |
| 3rd generation (ceftizoxime) | 103 | 77.931 ± 12.62 | |
| Height (cm) | 0.457 | ||
| 1st generation (cefazolin) | 408 | 164.52 ± 10.91 | |
| 3rd generation (ceftizoxime) | 103 | 163.51 ± 17.16 | |
| BMI (kg/m2) | 0.059 | ||
| 1st generation (cefazolin) | 408 | 27.7407 ± 4.7 | |
| 3rd generation (ceftizoxime) | 103 | 28.7057 ± 4.76 | |
| GFR (mL/min/1.73 m2) | 0.052 | ||
| 1st generation (cefazolin) | 83 | 79.7740 ± 24.52 | |
| 3rd generation (ceftizoxime) | 28 | 91.6971 ± 31.6 | |
| Stone burden (mm) | 0.52 | ||
| 1st generation (cefazolin) | 408 | 34.339 ± 14.33 | |
| 3rd generation (ceftizoxime) | 103 | 35.377 ± 16.68 | |
| Anesthesia time (minute) | 0.001 | ||
| 1st generation (cefazolin) | 408 | 88.51 ± 42.3 | |
| 3rd generation (ceftizoxime) | 103 | 58.06 ± 20.43 | |
| Operation time (minute) | 0.103 | ||
| 1st generation (cefazolin) | 408 | 43.93 ± 17.91 | |
| 3rd generation (ceftizoxime) | 103 | 40.69 ± 19.82 | |
| Dosage of postoperative analgesic (mg) | 0.000 | ||
| 1st generation (cefazolin) | 346 | 36.81 ± 35.62 | |
| 3rd generation (ceftizoxime) | 103 | 68.88 ± 82.07 | |
| Duration of postoperative analgesic (day) | 0.003 | ||
| 1st generation (cefazolin) | 408 | 1.45 ± 0.75 | |
| 3rd generation (ceftizoxime) | 103 | 1.20 ± 0.75 |
Abbreviation: GFR, glomerular filtration rate.
Furthermore, the general complication rate was significantly lower in the third-generation cephalosporin group (13.0%) compared to the first-generation cephalosporin group (31.4%) (OR: 3.06, 95% CI: 1.68 - 5.58, P = 0.000), as shown in Table 3. When comparing postoperative fever rates, the first-generation cephalosporin group had a higher incidence (15.0%, n = 61) compared to the third-generation cephalosporin group (2.8%, n = 3) (P = 0.001). After adjusting for confounding factors such as diabetes, underlying diseases, hypertension, and cardiovascular diseases, the odds ratio of postoperative fever in the first-generation cephalosporin group was 0.203 times lower than in the third-generation cephalosporin group, showing a statistically significant difference (P = 0.043).
| Complication | 1st Generation (Cefazolin) (n = 408) | 3rd Generation (Ceftizoxime) (n = 103) | Odds Ratio (95% CI) | P-Value |
|---|---|---|---|---|
| Post-operative fever | 6.1 (1.89 - 20.07) | 0.001 | ||
| No | 347 (85.0) | 105 (97.2) | ||
| Yes | 61 (15.0) | 3 (2.8) | ||
| UTI after PCNL | 1.26 (1.21 - 1.32) | 0.607 | ||
| No | 407 (99.8) | 108 (100.0) | ||
| Yes | 1 (0.2) | 0 (0.0) | ||
| Pyelonephritis | 1.26 (1.21 - 1.32) | 0.607 | ||
| No | 407 (99.8) | 108 (100.0) | ||
| Yes | 1 (0.2) | 0 (0.0) | ||
| Sepsis | 1.26 (1.21 - 1.32) | 0.607 | ||
| No | 407 (99.8) | 108 (100.0) | ||
| Yes | 1 (0.2) | 0 (0.0) | ||
| Other | 3.06 (1.68 - 5.58) | 0.000 | ||
| No | 280 (68.6) | 94 (87.0) | ||
| Yes | 128 (31.4) | 14 (13.0) |
Abbreviations: PCNL, percutaneous nephrolithotomy; UTI, urinary tract infection.
a Values are expressed as No. (%).
However, no significant differences were observed between the two groups in terms of UTI, pyelonephritis, sepsis, or the success rate of surgery (P = 0.607), as detailed in Table 3.
According to Table 4, there was a significant difference in the duration of postoperative hospitalization between the two groups. Patients in the first-generation cephalosporin (cefazolin) group had an average hospitalization duration of 4.03 ± 1.57 days, while those in the third-generation cephalosporin (ceftizoxime) group had a significantly shorter duration of 1.31 ± 1.18 days (P = 0.000).
| Variables and Study Groups | Number | Mean ± SD | P-Value |
|---|---|---|---|
| Duration of return to normal life (day) | 0.001 | ||
| 1st generation (cefazolin) | 164 | 8.15 ± 2.93 | |
| 3rd generation (ceftizoxime) | 34 | 5.97 ± 3.37 | |
| Duration of postoperative hospitalization (day) | 0.000 | ||
| 1st generation (cefazolin) | 408 | 4.032 ± 1.57 | |
| 3rd generation (ceftizoxime) | 103 | 1.315 ± 1.18 |
Furthermore, the time taken to return to normal life also differed significantly between the two groups. The first-generation cephalosporin group had an average return-to-normal-life duration of 8.15 ± 2.93 days, whereas the third-generation cephalosporin group experienced a shorter duration of 5.97 ± 3.37 days (P = 0.001). These findings indicate that patients in the third-generation cephalosporin group had a quicker recovery and shorter hospital stay compared to those in the first-generation cephalosporin group.
5. Discussion
The results of this study indicate that the incidence of postoperative fever was significantly higher in patients who used the first-generation prophylactic antibiotic compared to the third-generation group (15.0% vs. 2.8%). However, there were no differences in the rates of UTI after surgery, pyelonephritis, and sepsis between the third- and first-generation cephalosporin groups. It is worth noting that significantly lower rates of complications were found in the third-generation cephalosporin group. Additionally, the study findings revealed higher rates of other surgical complications, such as transfusion or hemichorea, in patients who used the first-generation prophylactic cephalosporins. The surgical complication rates in patients undergoing PCNL who used first-generation prophylactic cephalosporins were significantly higher compared to the third-generation group (31.4% vs. 13.0%).
In a previous study conducted by Bae et al. (19), it was found that there were no differences in postoperative surgical complications between the two groups that used prophylactic third-generation and first-generation cephalosporins. However, they did find that the incidence of surgical site infections was significantly lower in the first-generation prophylaxis group (5.7%) compared to the third-generation prophylaxis group (16.5%). Additionally, they observed that the prevalence of infectious gram-positive bacteria was higher than that of gram-negative bacteria (67% vs. 23%) (19).
In a study conducted by Beam et al. (20), it was found that there was a significant difference in the dosage and usage of different third-generation and first-generation cephalosporins. They discovered that a single 1-gram dose of the third-generation cephalosporin ceftriaxone was not only effective and safe, but it also showed higher penetration into the tissue compared to multiple doses of the first-generation cephalosporin cefazolin (20).
In a study by Bratzler et al. (21), it was cautioned that the use of prophylactic antibiotics that are not in line with guidelines may be less effective in reducing infection. Furthermore, the use of antibiotics with excessively broad antimicrobial ranges could potentially lead to the emergence of new multidrug-resistant strains of bacteria. However, Bratzler et al. (21) recommended the use of narrow antibiotic ranges and older antibiotics as a selection for prophylactic antibiotics. This recommendation was based on factors such as cost, half-life, safety, and antibiotic resistance. They advised against the use of newer and broader-range antibiotics, as they may contribute to increased tolerance (21).
It is evident that PCNL, a surgical intervention for removing large kidney stones, can be aggressive and may introduce infectious germs to the wound site and inside the body. While the role of gram-positive bacteria like Staphylococcus aureus and Staphylococcus epidermidis in infections cannot be ignored, multidrug-resistant gram-negative bacteria such as Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus mirabilis, Acinetobacter species, Enterobacter species, and Citrobacter species may play a more significant role in postoperative infections. These gram-negative bacteria are known for their resistance to multiple antibiotics, complicating treatment and increasing the risk of complications (22).
Recent studies have shown that newer and broader-range third-generation cephalosporins offer advantages in reducing general surgical complications and fever rates in PCNL surgery. Given the increasing prevalence of resistant gram-negative bacteria and the antimicrobial characteristics of third-generation cephalosporins like ceftriaxone, it is believed that using these antibiotics in combination with aminoglycosides may provide excellent antimicrobial activity and a synergistic effect against gram-negative bacteria as a prophylactic measure. However, it is important to consider that some bacteria have intrinsic resistance to first-generation cephalosporins, which should not be overlooked when selecting the appropriate antibiotic.
The duration of postoperative hospitalization was found to be significantly shorter in patients who received third-generation prophylactic antibiotics compared to those who received first-generation antibiotics. Additionally, patients who received third-generation antibiotics had a significantly faster return to normal life than those who received first-generation antibiotics, a statistically significant difference. Furthermore, the consumption of postoperative analgesics was significantly lower in the third-generation group compared to the first-generation group. These findings suggest that prophylaxis with third-generation cephalosporins, along with aminoglycosides, in patients undergoing PCNL may have a positive impact, leading to decreased hospitalization duration, faster return to normal life, reduced general complications, lower rates of postoperative fever, and decreased consumption of postoperative analgesics.
The results of the current study indicate that administering ceftizoxime along with aminoglycosides as a prophylactic antibiotic 30 minutes prior to surgery can effectively reduce early postoperative fever rates, shorten hospitalization length, and expedite the return to normal life for patients undergoing PCNL surgery. This finding is significant given the potential risks associated with indiscriminate antibiotic use and the growing threat of antibiotic-resistant bacteria, particularly gram-negative strains, within our population. The combination of third-generation cephalosporins and aminoglycosides seems to have a beneficial effect in preventing postoperative fever and facilitating a quicker recovery for patients.
5.1. Limitations of the Study
This study had several limitations. Firstly, some participants may have provided inaccurate information regarding their return to normal life, which could have influenced the results. Secondly, the study was cross-sectional, spanning a 10-year period, which makes it difficult to establish a direct causal relationship between the use of prophylactic antibiotics and the outcomes observed. Thirdly, the research was conducted in only three hospitals within Guilan province, specifically in Rasht city, which limits the generalizability of the findings to the broader population. Lastly, it is important to note that early postoperative fever is often caused by tissue damage and inflammation, while infection-related postoperative fever typically occurs after 4 days. This distinction should be taken into account when interpreting the results of the study.