1. Background
Fetal hydronephrosis (HN) is one of the most commonly observed disorders in prenatal ultrasounds, affecting approximately 1 - 5% of pregnancies (1, 2). While about 98% of cases of mild HN improve without intervention, surgical intervention is necessary for almost all cases of severe HN (3). Researchers have employed proteomics technologies to identify novel biomarkers for acute kidney injury and chronic kidney disease. Metabolite profiles in blood and urine can serve as early indicators of kidney disease based on their unique biochemical signatures (4, 5), and metabolite analysis using methodologies such as LC-MS/MS holds significant promise for diagnosing and managing these conditions.
Although research on the blood and urinary peptidome has shown considerable potential, the study of the blood and urinary metabolome remains relatively under-explored, despite its capacity to provide a more comprehensive understanding of kidney function. Current advancements in blood and urinary omics face limitations when utilizing single-omics traits to accurately predict disease outcomes, particularly due to the "gray zone" where prediction accuracy is uncertain. To enhance diagnostic and prognostic capabilities, a more integrated approach that combines multiple omics data may be necessary (6).
While there is a growing body of research on neonatal HN, the precise mechanisms contributing to its development and progression remain elusive. One metabolite that plays a vital role in kidney function is L-arginine. L-arginine is a semi-essential amino acid crucial for various physiological processes, including the development and functioning of the kidneys (7). Research suggests that L-arginine may significantly impact kidney malformations and contribute to their prevention (8). However, its specific role in the context of neonatal HN has not been thoroughly investigated.
2. Objectives
This study is novel in its focus on quantifying arginine levels in neonates with HN. The aim of our research is to explore the association between arginine levels and the severity of neonatal HN within a cohort of affected infants. By comparing arginine concentrations in infants with HN to those in a healthy control group, we seek to enhance understanding of this condition and potentially identify new therapeutic targets.
3. Methods
3.1. Study Design and Population
This prospective case-control study was conducted on neonates with HN less than 44 weeks post-conceptional age, admitted to the NICU or neonatal ward of the Children's Medical Center in Tehran, Iran, from October 2021 to October 2023. Healthy neonates were referred to the child growth and development center for metabolic screening between days 3 to 5 of life. Neonatal HN was defined based on the society of fetal urology (SFU) grading system: SFU grade ≥ I or kidney anteroposterior diameter (APD) ≥ 7 mm; SFU grade ≥ II or kidney APD between 10 - 15 mm; and SFU grade ≥ III or kidney APD ≥ 15 mm (9).
Participants were excluded if they had congenital malformations, metabolic disorders, seizures, were receiving total parenteral nutrition, had been nothing by mouth (NPO) for more than 72 hours, or if there was a lack of parental consent. A single drop of blood was collected from the heels of neonates and placed on dry filter paper (Whatman 903) for metabolic analysis. Urine samples were also collected from the infants' post-breastfeeding during their hospitalization, using sterile pediatric urine collection bags. Urine and blood samples from healthy control newborns were collected using the same methods at the child growth and development center.
All samples were transported on ice blocks and stored at -40°C in the laboratory. The device measurement is based on the mass-to-charge ratio of the analyte (m/z), and the scanning mode utilized is multiple reaction monitoring (MRM). We transferred three 3.2 mm punches of the dried blood spots (DBS) sample, equivalent to 10 µL of plasma, into a 1.5 mL microtube. Following this, we added 200 µL of an internal standard solution containing amino acids and acylcarnitines from Chrome System and shook the mixture for 30 minutes at 600 RPM.
Next, we transferred 150 µL of the supernatant into a glass vial and evaporated it for 20 minutes at 45°C under a stream of nitrogen gas. We then added a solution of acetyl chloride and 1-butanol from Chrome System at a ratio of 1:9, totaling 50 µL. After thoroughly shaking the vial for one minute, we incubated it at 65°C for 15 minutes. The sample was then re-incubated at 45°C under a nitrogen gas atmosphere until it evaporated completely. Subsequently, we mixed the residue with 100 µL of a 75% acetonitrile solution from Chrome System and shook it for 10 minutes. Finally, 10 µL of the resulting mixture was injected into the SCIEX 3200 LC-MS/MS machine.
3.2. LC-MS/MS Calibration
Prior to sample analysis, the LC-MS/MS system, model SCIEX 3200, was calibrated using a polypropylene glycol (PPG) solution to ensure the accuracy of the mass-to-charge ratio (m/z). The PPG solution, which contains molecules with known masses, was injected into the system to verify the proper functioning of the mass spectrometer. Calibration was performed regularly in accordance with the manufacturer's instructions to maintain the precision and accuracy of the device throughout the study. This process included adjustments for any mass deviations and ensured the stability of the device's sensitivity in detecting target analytes.
To further ensure accuracy and evaluate device performance, low and high controls provided by Chrom Systems were also employed. These controls, designed to assess the device's performance at both low and high concentration levels, were regularly utilized throughout the analyses. By using these controls, we were able to detect any deviations or changes in device performance, ensuring the analyses conducted were precise and reliable. Implementing these controls enhanced the accuracy of the results and confirmed the optimal functioning of the device.
3.3. Sample Size
We aimed for a power of 95% and, based on previous studies and expert opinion, estimated an effect size of 0.25 (indicating a large difference in arginine levels between the healthy population and those with HN). Using this information, along with a 95% confidence interval and an α error of 0.05, we calculated a total sample size of 42 (21 cases and 21 controls). To account for an anticipated attrition rate of approximately 15%, we adjusted the final sample size to 48 participants (24 in each group). The sample size was calculated using G*Power 3.1 software.
3.4. Statistics
The normality of the data was assessed using the Kolmogorov-Smirnov test. For data that exhibited a normal distribution, continuous variables were expressed as means and standard deviations, while categorical variables were summarized as frequencies. The Pearson correlation coefficient was calculated to evaluate the relationship between two quantitative variables. Data analysis was performed using GraphPad Prism version 8.3.0 for Windows (GraphPad Software, La Jolla, CA, USA).
3.5. Ethics
The project adhered to ethical principles and complied with national norms and standards for conducting medical research in Iran. It received approval from the Ethics Committee of Tehran University of Medical Sciences, with the ID: IR.TUMS.CHMC.REC.1400.147.
4. Results
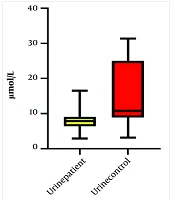
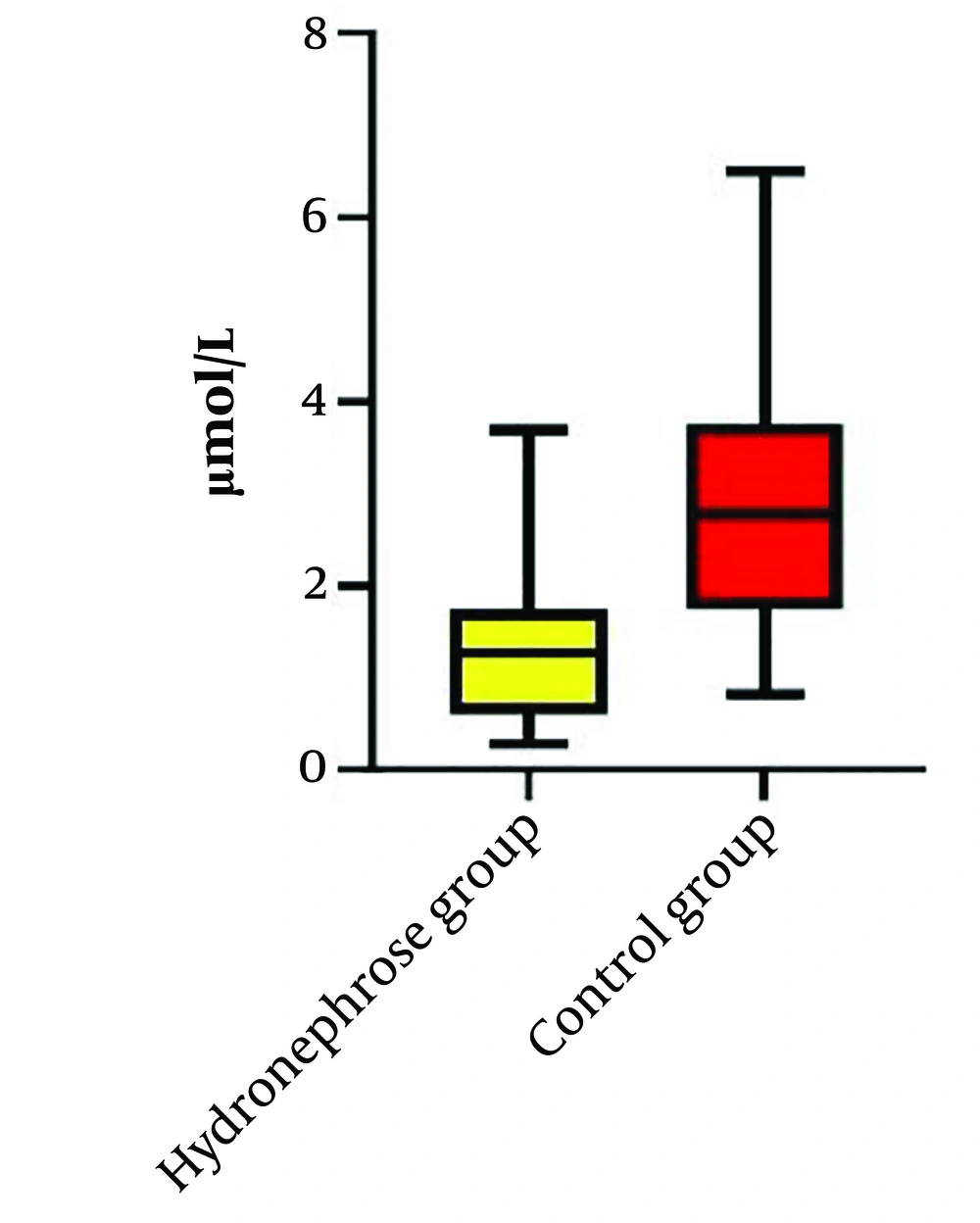
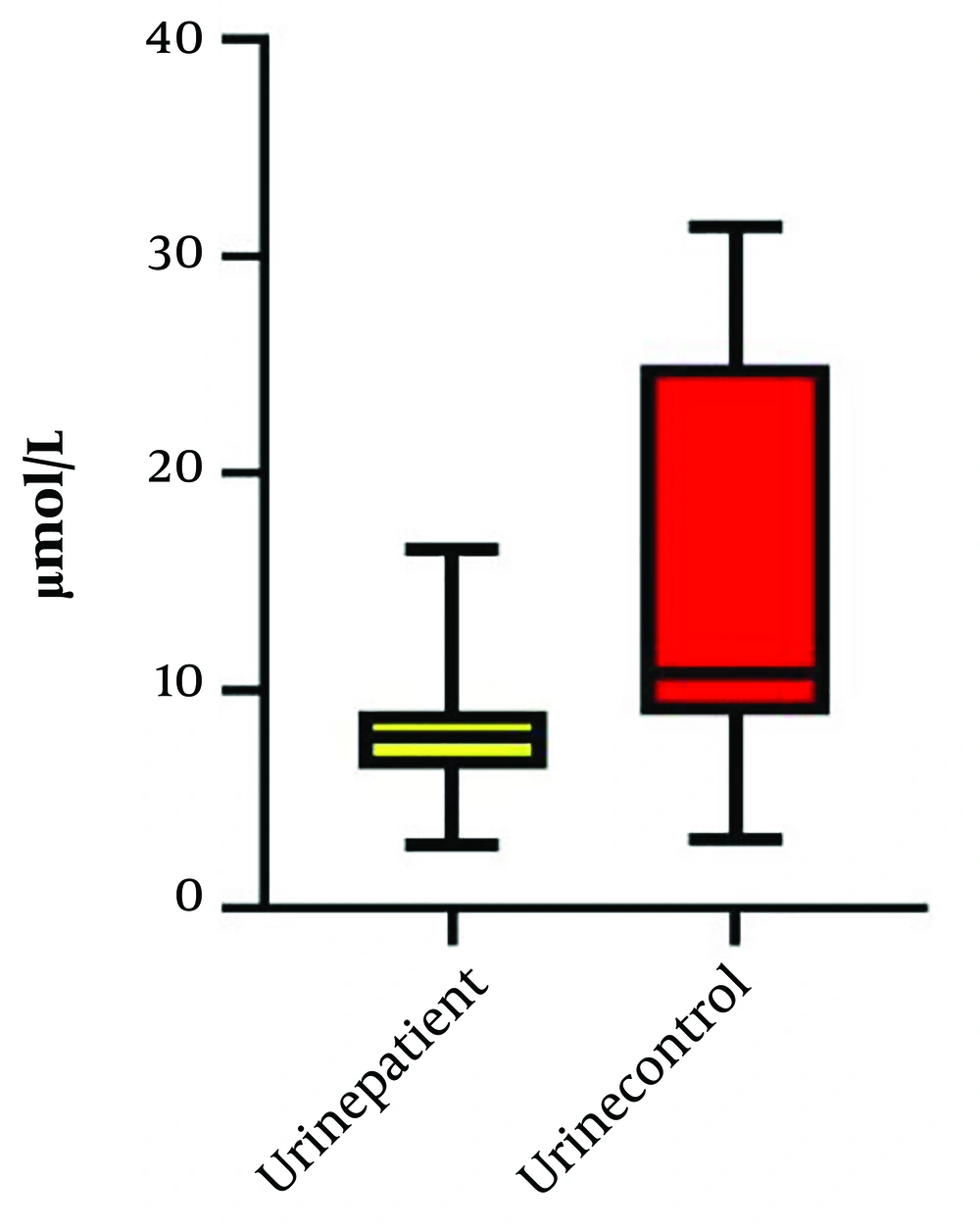
Our study compared the levels of arginine in the plasma and urine of neonates with HN and healthy neonates. A total of thirty-five patients with HN were initially included in the study; however, seven of them did not meet the inclusion criteria due to multiple anomalies, and the data for four patients were incomplete. Ultimately, twenty-four patients with HN and twenty-four healthy neonates were enrolled in the control group. Table 1 presents the demographic, laboratory, and clinical data of the HN group. The most common cause of HN observed was the posterior urethral valve (PUV), as shown in Table 2. The levels of urine and serum arginine in the control group were found to be higher than those in the HN group. This difference was significant for urine levels (P = 0.03) but not for serum levels (Table 3). A Pearson’s correlation analysis was conducted to evaluate the relationship between plasma arginine levels in the HN group and arginine levels in the control group. The results indicated a non-significant positive relationship between plasma arginine levels and control arginine levels, [r (24) = 0.048, P = 0.82] (Figure 1). In contrast, a Pearson's correlation between urine arginine levels in the HN group and arginine levels in the control group revealed a significant positive relationship, [r (24) = 0.44, P < 0.05] (Figure 2). We did not find any significant relationship between plasma and urine arginine levels and the right and left kidney measurements, as well as with APD, BUN, and creatinine levels. We performed a bootstrap analysis on the urine samples, which yielded a mean difference of 0.03. The estimated 95% confidence interval (CI) was (0.15, 0.21), and the P-value was 0.45.
| Variables (N = 24) | Values a | 95% CI (Lower Bound - Upper Bound) |
|---|---|---|
| Birth weight (g) | 2971 ± 519 | 2751 - 3190 |
| Gestational age (wk) | 37.4 ± 2 | 36.4 - 38.3 |
| Delivery route (C/S) | 19 (79.2) | - |
| Gender (male) | 23 (95.8) | - |
| Bun (mg/dL) | 19.57 ± 24.5 | 9.28 - 30.3 |
| Cr (mg/dL) | 0.85 ± 0.1 | 0.63 - 1.07 |
| Na (meq/L) | 136.5 ± 1.1 | 134 - 139 |
| K (meq/L) | 4.5 ± 0.1 | 4.2 - 4.8 |
| SG | 1008 ± 1.09 | 1005 - 1010 |
| Pelvic right diameter (mm) | 8.8 ± 1.1 | 6.56 - 11.19 |
| Pelvic left diameter (mm) | 10.7 ± 5.4 | 8.31 - 13.17 |
a Values are expressed as mean ± SD or No. (%).
| Variables (N = 24) | Frequency (%) |
|---|---|
| PUV | 11 (45.8) |
| UPJO | 3 (12.5) |
| ANHN | 6 (25) |
| Ectopic urethra | 1 (4.2) |
| Urinary tract infection | 2 (8.3) |
| Urethrocele | 1 (4.2) |
Abbreviations: PUV, posterior urethral valves; UPJO, ureter pelvic junction obstruction; ANHN, antenatal hydronephrosis.
Abbreviation: HN, hydronephrosis.
a Values are expressed as mean ± SD.
The results of the study indicated that surgery was performed on 13 patients (54.2%), while 11 patients (45.8%) were managed with observation. We conducted a receiver operating characteristic (ROC) analysis to evaluate the need for surgery, which revealed that the test had low sensitivity, evidenced by an area under the curve (AUC) of 0.53 (95% confidence interval: 0.29 - 0.77, P = 0.7).
5. Discussion
This study found that both urine and serum arginine levels in the HN group were lower compared to those in healthy neonates. However, no significant relationships were observed between arginine levels and the anteroposterior (AP) pelvic diameter or the necessity for surgical intervention. Conversely, the research conducted by Begou et al. demonstrated that levels of several biomarkers, including arginine, decreased in correlation with both the AP pelvic diameter and the likelihood of requiring surgery (10). Arginine is a precursor to nitric oxide (NO), a crucial molecule involved in kidney function, particularly during injury (8). The production of NO by endothelial constitutive nitric oxide synthase (cNOS) within the kidney helps regulate glomerular microcirculation by affecting the tone of the afferent arterioles and mesangial cells. Additionally, both the macula densa and the afferent arterioles play significant roles in regulating glomerular hemodynamics through tubuloglomerular feedback mechanisms and renin secretion. A deficiency in NO production can lead to hypertension and potential damage to the glomeruli (11). This deficiency may arise from either limited availability of the substrate L-arginine or increased levels of endogenous inhibitors of NO synthase, such as asymmetric dimethylarginine (ADMA) (12). Interestingly, the study by Begou et al. reported low ADMA levels in the ureteropelvic junction obstruction (UPJO) group that required surgery (10). Cherla and Jaimes noted that exogenous L-arginine can have protective effects in certain kidney diseases, suggesting potential therapeutic avenues (8). Buffin-Meyer’s research utilized various fetal urinary metabolomes to predict postnatal kidney outcomes, indicating the importance of metabolomic profiles in this context (13). Similarly, the metabolomics analysis by Aurélien Scalabre et al. highlighted the effectiveness of these profiles in the early identification of transient dilatations that may necessitate surgical intervention for UPJO (14).
To improve the early detection and severity assessment of kidney abnormalities, it is imperative that we identify a cost-effective biomarker. Urinary arginine may play a role in predicting kidney malformations; however, further research with larger sample sizes is necessary to reach more definitive conclusions. One limitation of our study is the inconclusive association between arginine levels and clinical outcomes, such as the need for surgery, which restricts the immediate clinical application of our findings. While we established a significant difference in urine arginine levels between the HN and control groups, the clinical implications of this finding require further investigation to enhance its utility in clinical practice. This limitation can be attributed to the small sample size, as the diagnostic kits used were quite expensive, and we did not receive funding from the university. Additionally, given that this was a residency thesis conducted within a limited timeframe, the occurrence of obstructive hydronephrosis leading to surgery was rare. These factors collectively contributed to the low sample size in our study.
5.1. Conclusions
In summary, arginine levels in urine and serum may be lower in neonates with HN compared to healthy neonates, suggesting a potential relationship between arginine and kidney function. However, larger studies involving more cases are necessary to establish definitive conclusions regarding the role of arginine in predicting kidney malformations. Identifying a cost-effective biomarker for the early detection of kidney abnormalities remains a critical objective in pediatric nephrology.