1. Context
Nocturnal enuresis is a common complaint experienced by children. Nocturnal enuresis is defined as incontinence that occurs only during sleep and is experienced for at least three consecutive months in children aged over 5 years. The prevalence of nocturnal enuresis decreases with age: Around 15% of 5-year-old children experience nocturnal urinary incontinence, while only about 5 - 10% experience it at 7 years old, and 1 - 2% at ≥ 15 years old. It is known that spontaneous resolution of nocturnal enuresis occurs in up to 15% (1, 2).
In classification, nocturnal enuresis is divided into monosymptomatic nocturnal enuresis (MNE) and non-monosymptomatic nocturnal enuresis (NMNE). Monosymptomatic nocturnal enuresis is intermittent nocturnal incontinence without lower urinary tract symptoms. On the other hand, NMNE is nocturnal enuresis accompanied by lower urinary tract symptoms. Generally, enuresis can have a negative impact on a child's psychosocial development. Enuresis can also be associated with various other comorbidities such as learning disorders, neurological diseases, attention-deficit/hyperactivity disorder, and sleep disorders (1). Findings from a meta-analysis conducted by Cai et al. in 2023 showed that combination therapy was superior to desmopressin monotherapy in treating nocturnal enuresis. However, while this meta-analysis examined nocturnal enuresis as a whole, our meta-analysis will focus specifically on MNE (3).
Desmopressin, a synthetic analog of vasopressin, has been used for decades to manage nocturnal enuresis and has proven to be effective. Desmopressin works by reducing urine volume and intravesical pressure during the night. In conditions of high nighttime diuresis, desmopressin administration has shown success rates of up to 70%. However, some patients may show resistance to desmopressin or suspicion of having overactive bladder at night, so desmopressin administration can be combined with anticholinergics.
2. Objectives
3. Data Sources
3.1. Search Strategy and Selection Criteria
This review adhered to the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines. We conducted literature searches using electronic databases: Science Direct, Scopus, Cochrane, Embase, PubMed, as well as manual searching with the following keywords (Table 1). Literature searching was performed from June to August 2023. Studies were considered eligible for inclusion if they were conducted in English or Indonesian, had full-text availability, were clinical trials, participants were aged 0 - 18 years, and the studies evaluated the comparison between desmopressin and anticholinergic treatment versus desmopressin alone in pediatric patients with MNE. Articles were excluded if their type was unsuitable, such as abstracts, reviews, comments, or similar types.
| Databases | Search Keywords |
|---|---|
| Scopus | (Nocturnal Enuresis) AND (Desmopressin) AND (anticholinergic) |
| Science Direct | (Nocturnal Enuresis) AND (Desmopressin) AND (anticholinergic) |
| Cochrane | (Nocturnal Enuresis) AND (Desmopressin) AND (anticholinergic or oxybutynin or hyoscyamine) |
| Embase | (Nocturnal Enuresis) AND (Desmopressin) AND (anticholinergic OR oxybutynin OR hyoscyamine) |
| PubMed | (Nocturnal Enuresis) AND (Desmopressin) AND (anticholinergic) |
| Hand searching | (Nocturnal Enuresis) AND (Desmopressin) AND (anticholinergic) |
3.2. Definition of Intervention and Outcomes
We defined the control group as patients receiving only desmopressin, while the intervention group consisted of patients receiving a combination of desmopressin and anticholinergic treatment. The primary outcome assessed both complete remission (90 - 100% reduction in mean wet nights compared to baseline) and partial remission (50 - 90% reduction), with the latter being considered as part of total remission, which is the sum of complete and partial remission. Meanwhile, the secondary outcome assessed the adverse effects.
3.3. Study Selection and Data Extraction
Three reviewers independently screened the titles and abstracts of studies identified through database searching and assessed the eligibility of each study based on inclusion and exclusion criteria. The chosen studies were then subjected to data extraction, which involved collecting the following information: (1) First author’s surname; (2) publication year and location; (3) study design; (4) age of the population included in the study; (5) entry time of the population into the study; (6) intervention given (drug and dose); (7) outcomes at 1 month and 3 months; (8) side effects.
3.4. Quality Appraisal
The risk of bias was evaluated using tools provided by Cochrane. These evaluations were performed independently by all authors (M.A.I.M., N.A.T., and F.A.R.). Each study included was classified as “low-risk,” “high-risk,” or “unclear risk” based on Cochrane risk-of-bias domains. If there was any discrepancy regarding the bias assessment, it was resolved by discussion among all the authors.
3.5. Statistical Analysis
A forest plot was generated using Review Manager 5.4. Risk ratio (RR) was used to evaluate primary outcomes in included studies, such as the incidence of remission (at month 1 and month 3 after treatments). All meta-analyses were performed using random-effects models. Heterogeneity of the study was evaluated with I² statistics. When the I² value was greater than 50%, this indicated a high level of heterogeneity. If the P-value was < 0.05, the result was considered statistically significant. Side effects were only analyzed descriptively.
4. Results
4.1. Selection of Studies and Study Characteristics
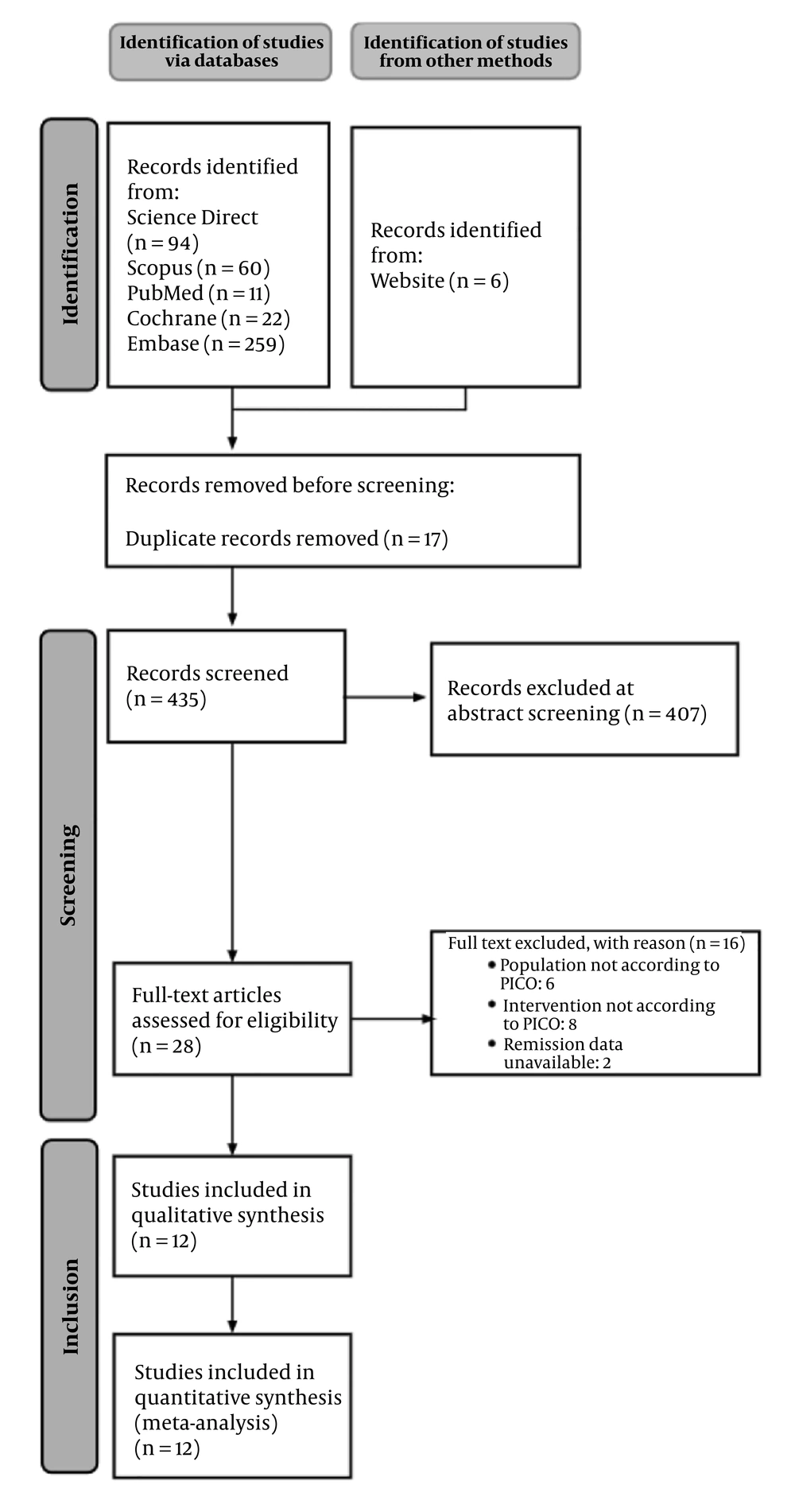
From database searching, 446 publications were identified. From manual searching, 5 additional publications were also identified. After duplication removal, 434 articles remained for initial screening. Selected articles were the result of agreement between 3 authors (M.A.I.M., N.A.T., and F.A.R.). Four hundred seven articles were excluded as they did not meet eligibility criteria based on the title and abstract. For the remaining 27 studies, 6 studies were excluded because the population did not meet eligibility criteria, 8 studies were excluded because the intervention did not meet eligibility criteria, and 2 studies were excluded because the desired outcome was not available. In total, 16 studies were excluded. The final screening yielded 11 clinical trials that met the eligibility criteria and were then included for analysis. The PRISMA flow diagram of our study can be seen in Figure 1.
Included studies in this meta-analysis were published between 2006 and 2021, with a total of 872 pediatric patients. The therapies for each study consisted of two groups, where one group was treated with desmopressin (either orally or nasally) and the other was treated with a combination of desmopressin and anticholinergic agents, such as oxybutynin, tolterodine, solifenacin, or propiverine. A summary of the characteristics of the included studies is shown in Table 2.
| Studies (Year) | Location | Age | Entry Time | Intervention (Drug, Dose) | Number (Intervention/ Control) |
|---|---|---|---|---|---|
| Gozukucuk et al., 2021 (6) | Hisar Intercontinental Hospital, Dogus University, Istanbul, Turkey | 6 - 16 | Begin a | (A) Initial dose desmopressin 120 mg; non-responsive patients --> dose doubled (240 mg) after two weeks; (B) maximum dose desmopressin + 5 mg oxybutynin --> oxybutynin increased to max dose (10 mg), with 2.5 mg increases once in two weeks | 183 (92/91) |
| Ghanavati et al., 2021 (7) | Imam Khomeini Hospital in Ahwaz | 5 - 15 | Begin a | (A) One puff of desmopressin nasal spray; (B) one puff of desmopressin nasal spray + 2 mg tolterodine; (C) one puff of desmopressin nasal spray + 5 mg solifenacin | 62 (22 (A)/ 20 (B)/ 22 (C)) |
| Shim et al., 2021 (8) | Hallym University Sacred Heart Hospital Pediatric Urology Clinic | 6 - 14 | Begin a | (A) Desmopressin lyophilisate (MELT) 120 µg only; (B) desmopressin lyophilisate (MELT) 120 µg plus propiverine 5 mg. | 99 (50/49) |
| Kazi et al., 2020 (9) | Bahria University Medical & Dental College (PNS Shifa Hospital) and the National Institute of Child Health, Karachi | 7 - 13 | Begin a | (A) Zero point two mg desmopressin tab +5 mg oxybutynin; (B) zero point two mg desmopressin | 84 (42/42) |
| Ravanshad et al., 2017 (10) | Ghaem Hospital, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran | 5 - 15 | Begin a | (A) Desmopressin nasal spray (10 µm/each nostril) + oxybutinin 5 mg; (B) desmopressin nasal spray (10 µm/each nostril) | 59 (30/29) |
| Almusafer and Adel, 2017 (11) | University of Basrah | 6 - 14 | Begin a | (A) Twenty µm I/N; desmopressin + 5 mg oxybutynin, (B) Twenty µm I/N; desmopressin | 41 (20/21) |
| Kazemi Rashed et al., 2013 (12) | Tabriz University of Medical Sciences | 5 - 16 | Begin a | (A) Tolterodine 2 mg tablets and nasal Desmopressin; with a dose of 20 𝜇g; (B) placebo and nasal Desmopressin with a dose of 20 𝜇g | 99 (49/50) |
| Montaldo et al., 2012 (13) | Department of Pediatric Urology of the Second University of Naples | 6 - 13 | Failed b | (A) Two hundred forty μg desmopressin +; 5 mg Oxybutynin; (B) two hundred forty μg desmopressin + placebo | 120 (61/59) |
| Azhir et al., 2008 (14) | Alzahra hospital, Iran | 6 - 12 | Begin a | (A) Zero point one mg desmopressin; tab + 5 mg oxybutynin, (B) zero point one mg desmopressin; tab | 31 (10/21) |
| Austin et al., 2008 (15) | Washington University School of Medicine, St Louis Children’s Hospital, St Louis, Missouri | 6 - 17 | Failed b | (A) Desmopressin (0.6 mg; total per night) and placebo; (B) desmopressin (0.6 mg; total per night) and tolterodine LA (4 mg) | 34 (18/16) |
| Radvanska et al., 2006 (16) | Department of Pediatrics, Comenius University Medical School, Bratislava, Slovakia. | 6 - 15 | Failed b | (A) Twenty µg desmopressin; +; 5 mg oxybutynin; (B) twenty µg desmopressin | 60 (19/60) sequential |
| Park et al, 2014 (17) | Department of Pediatrics, Ajou University Hospital, Ajou University School of Medicine, Suwon, Republic of Korea | 5 - 16 | Begin a | (A) Desmopressin 0.2 mg daily and propiverine 10 mg daily; (B) desmopressin 0.2 mg daily | 84 (42/42) |
a Begin: No prior history of failed desmopressin therapy.
b Failed: Prior history of failed desmopressin therapy.
The included studies generally used remission (either complete or partial) as the primary outcome. Remission rates were typically measured at month 1 and month 3 post-treatment. The primary outcomes of the included studies can be seen in Table 3.
| Studies (Year) | 1 Month | 3 Months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete Remission | Partial Remission | Total Response | Complete Remission | Partial Remission | Total Response | |||||||
| D | I | D | I | D | I | D | I | D | I | D | I | |
| Gozukucuk et al., 2021 (6) | 65/91 | 69/92 | 6/91 | 10/92 | 73/91 | 79/92 | 70/91 | 80/92 | 5/91 | 6/92 | 75/91 | 86/92 |
| Ghanavati et al., 2021 (7) | 12/22 | B: 1, C: 17, total: 32/42 | NA | NA | NA | NA | 14/22 | B: 17, C: 19, total: 36/42 | NA | NA | NA | NA |
| Shim et al., 2021 (8) | 4/49 | 9/50 | 18/49 | 20/50 | 22/49 | 29/50 | 11/49 | 22/50 | 27/49 | 25/50 | 38/49 | 47/50 |
| Kazi et al., 2020 (9) | 13/42 | 13/42 | 27/42 | 29/42 | 40/42 | 42/42 | 36/42 | 42/42 | 5/42 | 0 | 41/42 | 42/42 |
| Ravanshad et al., 2017 (10) | 21/29 | 25/30 | NA | NA | NA | NA | 13/29 | 26/30 | NA | NA | NA | NA |
| Almusafer and Adel, 2017 (11) | 10/21 | 10/20 | 6/21 | 4/20 | 16/21 | 14/20 | 12/21 | 12/20 | 6/21 | 6/20 | 18/21 | 18/20 |
| Kazemi Rashed et al., 2013 (12) | 17/50 | 27/49 | 23/50 | 17/49 | 40/50 | 44/49 | NA | NA | NA | NA | NA | NA |
| Montaldo et al., 2012 (13) | 3/59 | 13/61 | 15/59 | 7/61 | 18/59 | 20/61 | NA | NA | NA | NA | NA | NA |
| Azhir et al., 2008 (14) | 1/21 | 3/10 | 1/21 | 3/10 | 2/21 | 6/10 | 5/21 | 7/10 | 11/21 | 2/10 | 16/21 | 9/10 |
| Austin et al., 2008 (15) | 1/16 | 3/18 | 4/16 | 5/18 | 5/16 | 8/18 | NA | NA | NA | NA | NA | NA |
| Radvanska et al., 2006 (16) | 21/60 | 19/19 | 20/60 | 0/19 | 41/60 | 19/19 | NA | NA | NA | NA | NA | NA |
| Park et al, 2014 (17) | 6/49 | 13/49 | 24/49 | 25/49 | 30/49 | 38/49 | 16/49 | 32/49 | 25/49 | 15/49 | 41/49 | 47/49 |
Abbreviations: NA, not available; D, desmopressin; I, intervention (combination); B, one puff of desmopressin nasal spray + 2 mg tolterodine; C, One puff of desmopressin nasal spray + 5 mg solifenacin.
Four out of 12 included studies reported side effects from both desmopressin and combination therapy. The most common side effects were constipation and dry mouth. Other side effects reported included allergic reactions, nausea, loss of appetite, headache, and nasal irritation. The summary of side effect occurrence can be seen in Table 4.
| Variables | Desmopressin | Combination |
|---|---|---|
| Allergic reaction | 7 (5) | 0 (0) |
| Nausea | 7 (5) | 1 (0.7) |
| Loss of appetite | 2 (1.4) | 16 (11.3) |
| Headache | 12 (8.5) | 2 (1.4) |
| Constipation | 26 (18.4) | 21 (14.8) |
| Dryness of mouth | 26 (18.4) | 21 (14.8) |
| Nasal irritation | 1 (0.7) | 2 (1.4) |
| Total population | 141 | 142 |
aValues are expressed as No. (%).
4.2. Risk of Bias
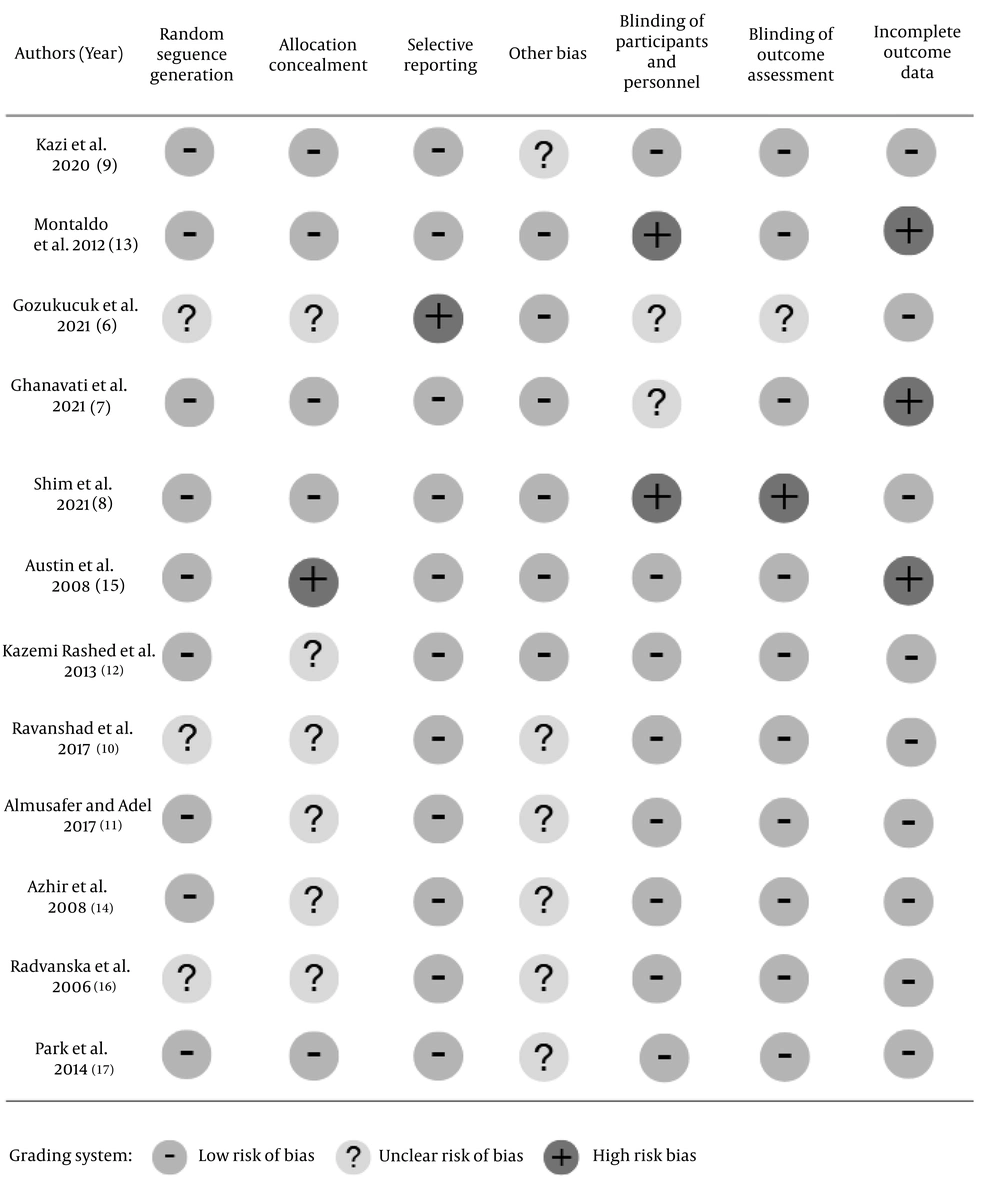
As shown in Figure 2, all of the studies included in our study were assessed using seven domains from the Cochrane risk of bias tool. The domains included were random sequence generation, allocation concealment, selective reporting, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and other bias. Risk of bias was assessed by all authors, and any differences between studies were discussed to reach a resolution. All authors participated in the risk of bias assessment and reached a consensus on the final assessment.
4.3. Quantitative Analysis
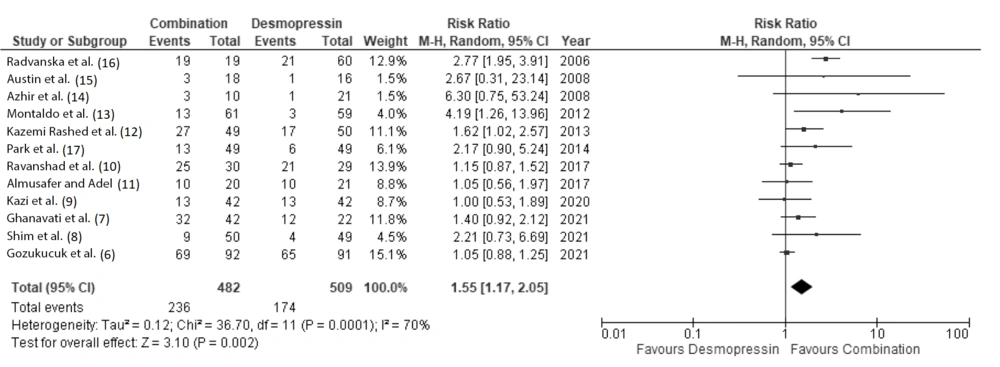
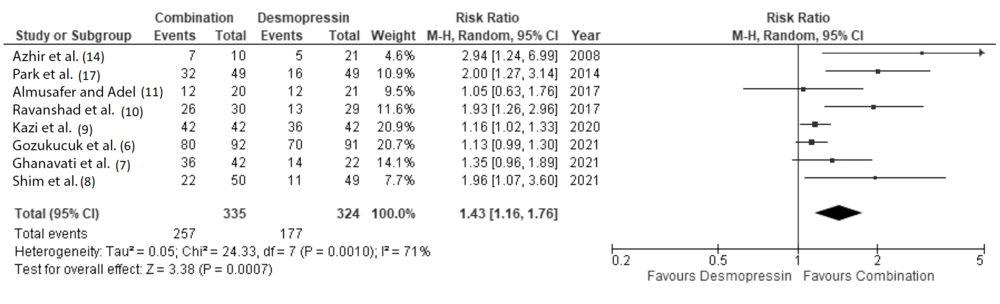
Eleven clinical studies, consisting of 872 pediatric patients, were included for quantitative analysis. Our meta-analysis showed that patients receiving combination therapy had a significantly higher probability of achieving complete remission at 1 month and 3 months after therapy, with a RR of 1.55 (95% CI: 1.17 to 2.05) and 1.43 (95% CI: 1.16 to 1.76), respectively (Figures 3 and 4).
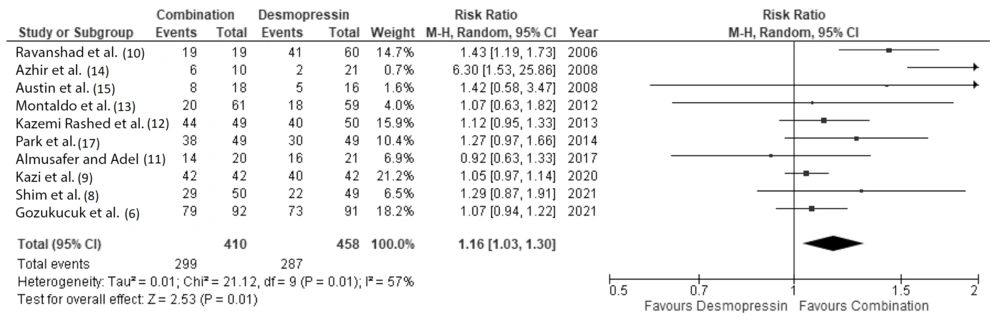
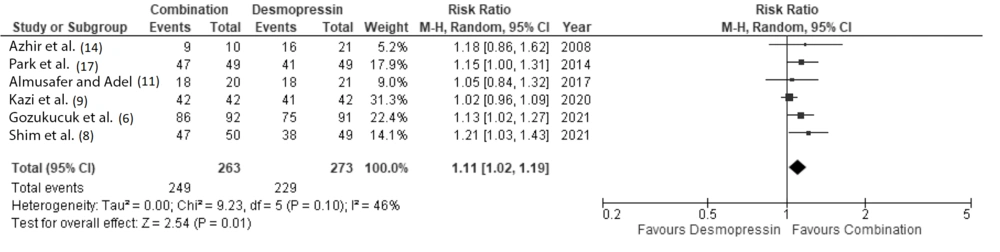
When we analyzed patients with complete and partial remission (summed up and referred to as total remission), our analysis also showed that patients receiving combination therapy had a significantly higher probability of achieving remission at 1 month and 3 months (RR 1.16 (95% CI: 1.03 to 1.3) and 1.11 (95% CI: 1.02 to 1.19), respectively (Figures 5 and 6).
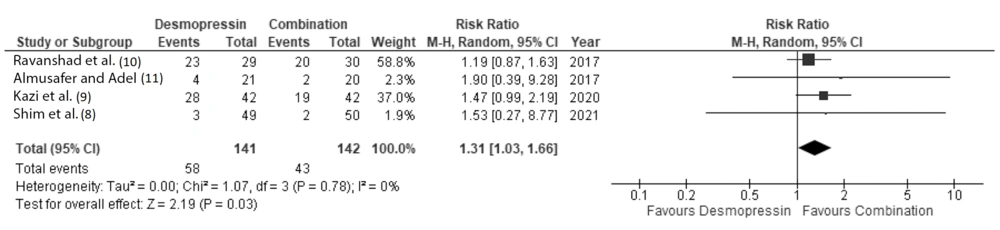
Similar side effects were observed in both the desmopressin group and the combination group. However, when conducting a meta-analysis of reported side effects, there was a slight but significant difference favoring combination therapy over desmopressin monotherapy in terms of adverse effects. The forest plot representing the reported side effects is shown in Figure 7.
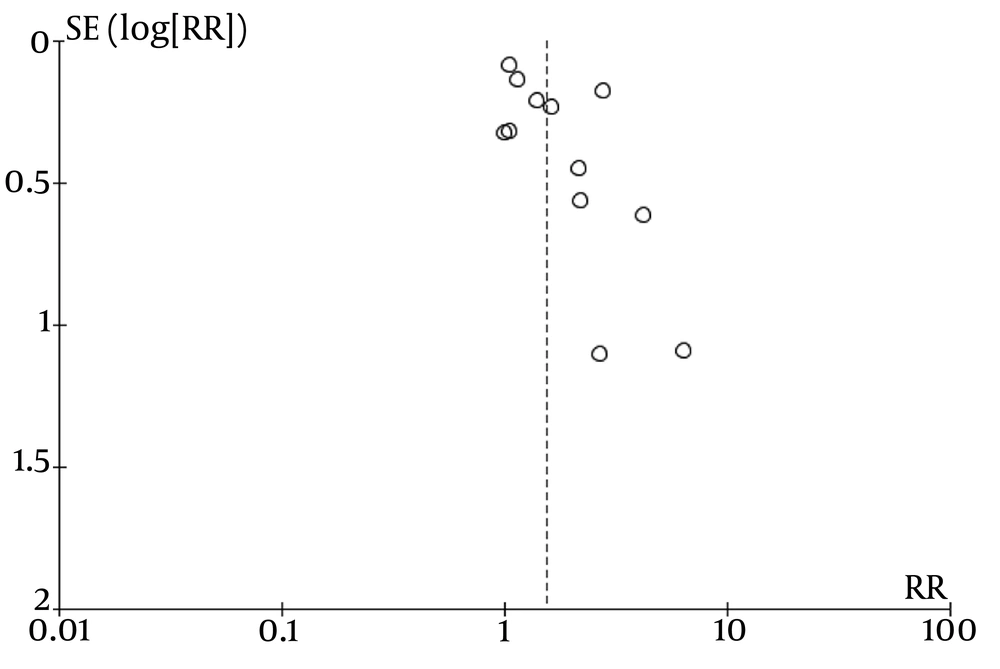
Our meta-analysis may have publication bias, as indicated by the asymmetry in the funnel plot analysis for all included studies. The funnel plot representing these studies is shown in Figure 8.
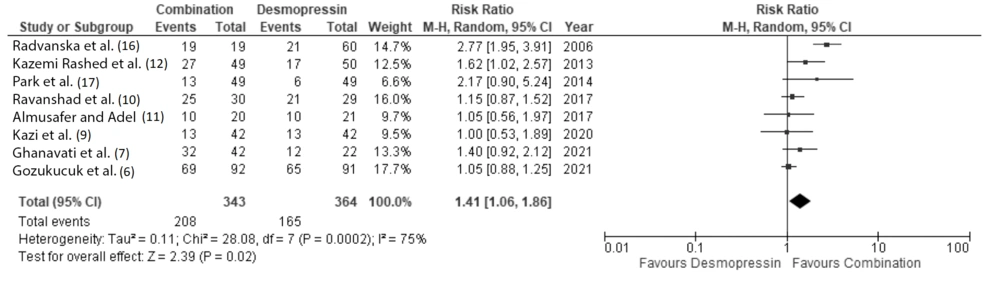
To address the potential for publication bias, we performed a sensitivity analysis by excluding studies with wide confidence intervals. Specifically, we excluded four studies with 95% confidence interval ranges greater than 5.00. Based on this sensitivity analysis, patients receiving combination therapy still had a significantly higher probability of achieving complete remission at 1 month after therapy, with a RR of 1.41 (95% CI: 1.06 to 1.86) (Figure 9).
5. Discussion
Our meta-analysis demonstrated that combination therapy of desmopressin and anticholinergics is significantly more effective in achieving remission for MNE pediatric patients compared to desmopressin monotherapy. At one month after therapy, the RR for complete remission and total remission favored the combination therapy, with a RR of 1.52 (95% CI: 1.14 - 2.02) and 1.15 (95% CI: 1.02 - 1.30), respectively. These findings suggest that pediatric patients receiving combination therapy are more likely to have reduced wet nights compared to those who only received desmopressin monotherapy. These results align with previous studies that have shown increased efficacy when these two pharmacological agents are combined (12, 13, 16).
One possible mechanism to explain the efficacy of combination therapy is the enhancing mechanisms of action between desmopressin and anticholinergics. Desmopressin, an analog synthetically made to mimic vasopressin, increases water reabsorption in the kidneys, thus resulting in reduced urine production at night. On the other hand, anticholinergics could reduce detrusor activity by inhibiting muscarinic receptors in detrusor muscles. By reducing urine production and decreasing detrusor overactivity, this could result in a more comprehensive treatment for MNE. In a study conducted by Azarfar et al., 59 patients with primary MNE were selected and divided into two groups: The first group received desmopressin and oxybutynin, and the second group received desmopressin and tolterodine. The results were satisfactory, although it showed that desmopressin plus tolterodine performed better than desmopressin plus oxybutynin (18).
Our results demonstrate the superiority of combination therapy for both clinical outcomes and adverse effects. However, our findings regarding side effects were contradicted by a meta-analysis performed by Cai et al. in 2023, which stated that the incidence of adverse reactions was not significantly different between the desmopressin and combination groups (3). The most common side effects reported were constipation and dryness of the mouth. In an article written by Ghossein et al., anticholinergics are well known to reduce gut motility by inhibiting muscarinic receptors in the gastrointestinal tract, thus resulting in constipation (19). Meanwhile, desmopressin could also affect the balance of electrolytes, and we know that electrolyte imbalance could accelerate the occurrence of constipation (20). Anticholinergics could also cause dry mouth by interrupting the neural stimulation of saliva secretion (21). Meanwhile, the effects of desmopressin on dryness of mouth need further studies.
5.1. Limitations
Our meta-analysis may be affected by publication bias, which could impact the overall results and the reliability of our findings. Publication bias might cause an overestimation of the true effect size. However, we conducted a sensitivity analysis, excluding studies with wide confidence intervals. This additional analysis allows us to confirm our findings and ensures that the effect size is not driven solely by potentially biased studies.
5.2. Guidelines for Future Research
Further research could focus on exploring the long-term benefits and identifying any additional potential side effects associated with desmopressin and anticholinergics. Additionally, a comprehensive cost-analysis should be conducted to calculate the economic feasibility, specifically of combination therapy with desmopressin and anticholinergics compared to desmopressin monotherapy. This research will provide more robust findings regarding the safety, efficacy, and cost-effectiveness profile, thereby supporting more effective clinical decision-making for MNE in the future.
5.3. Conclusions
Our results support the use of combination therapy consisting of desmopressin and anticholinergics as a viable option for treating MNE, especially in cases where monotherapy with desmopressin is insufficient. However, further research is still needed to uncover its long-term benefits and other potential side effects.