1. Context
In the last four decades, obesity has emerged as one of the most serious public health challenges worldwide (1). Studies in the European Union estimate that 30 - 70% of the adult population is overweight, with 10 - 30% classified as obese (2). The rate of overweight among African children under 5 years of age has increased by 24% since 2000, and in 2019, nearly half of Asian children under 5 were classified as obese or overweight (3). Obesity rates have increased significantly among both men and women across all age groups, with a proportionately higher prevalence in older individuals and women (4)
A study conducted in Sweden found that patients with obesity have a higher susceptibility to mental illness and experience a poorer quality of life compared to the general population (2). This susceptibility can be attributed to the presence of various coexisting diseases associated with obesity, which include not only an increased risk of cardiovascular disease and metabolic syndrome but also urological dysfunction (5, 6). Abdominal obesity results from the accumulation of body and visceral fat in the abdominal cavity, which in turn increases intra-abdominal and intravesical pressure. Consequently, this condition can lead to stress urinary incontinence (SUI), urge urinary incontinence (UUI), and overactive bladder (OAB) (7).
The International Continence Society and the International Urogynecological Association define OAB as urinary incontinence (UI), typically characterized by frequency and nocturia, which may be accompanied by UUI or occur without it, and is present in the absence of urinary tract infection or other evident pathologies (8, 9). The prevalence of OAB is estimated to range from 5 to 10% (10). Additionally, among young women aged 18 - 35 years, approximately 16% are overweight (11). Urgency is the key symptom in diagnosing OAB and is closely associated with a frequent daytime urge to urinate, nocturia, and incontinence (12).
Urinary incontinence is the involuntary leakage of urine (13). Urinary incontinence can affect individuals of any age, but it most commonly impacts men and women aged sixty years or older. Urinary incontinence can be classified in various ways based on the predominant symptom constellation and underlying pathophysiology. It may be categorized as (1) stress UI, (2) urgency UI, or (3) mixed UI, which includes symptoms of both stress and urgency UI. Other categories of UI include overflow incontinence, nocturnal enuresis, post-micturition dribble, continuous incontinence (often seen with a vesicovaginal fistula), and insensible incontinence, where individuals may be unaware of how it occurred (14). Urinary incontinence negatively impacts an individual’s quality of life. A systematic review found that OAB is associated with lower quality of life levels compared to other types of UI (15).
Considering the connection between weight gain and the occurrence of OAB, which significantly and negatively impacts quality of life, several treatment approaches have been developed to address OAB by targeting weight reduction. One study observed that, following bariatric surgery and subsequent weight loss, UI symptoms completely resolved in 33% of women after 6 months, with the highest success rate in the OAB group (54%), compared to 26% in the SUI group and 32% in the Mixed Incontinence group. Additionally, another study documented the sustained reduction of UI symptoms for up to 6 years post-bariatric surgery (16). Furthermore, another study documented that the reduction in UI symptoms was sustained for up to 6 years following bariatric surgery (17). Not only through surgery, but diet and exercise also demonstrated significant results after 6 months (18).
2. Objectives
The objective of this review is to evaluate the effects of weight loss programs, whether through surgery or dietary management, on reducing UI symptoms in obese and overweight female patients with OAB.
3. Data Sources
3.1. Eligibility Criteria and Study Selection
This systematic review adhered to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines established by Cochrane. Our objective was to evaluate the effect of weight loss programs on symptoms of OAB among women who are overweight or obese (aged ≥ 18 years).
The inclusion criteria for this study encompassed all clinical studies that assess the impact of weight reduction on OAB symptoms in overweight or obese women, incorporating both surgical and non-surgical weight loss methods. We excluded systematic reviews or meta-analyses, literature reviews, non-English articles, editorial letters, animal studies, and unpublished studies. Each author independently assessed the eligibility of the studies.
3.2. Literature Searching Strategy
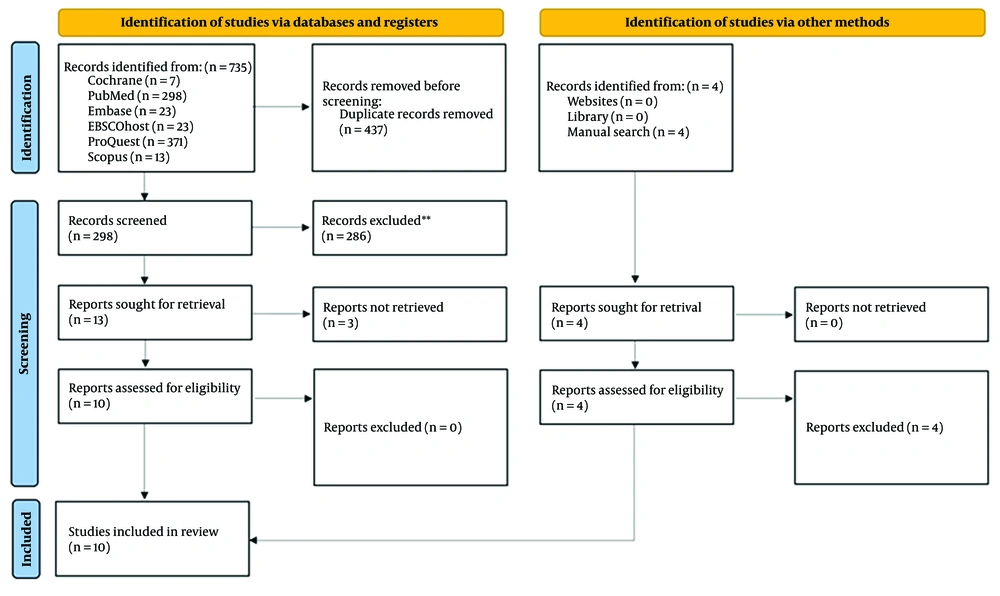
On June 11th, 2023, a comprehensive literature search was conducted across six electronic databases: (1) PubMed, (2) EBSCOhost, (3) ProQuest, (4) Embase, (5) Scopus, and (6) Cochrane, as illustrated in Figure 1. The following search strategy was employed: (Body weight reduction) OR (weight loss) OR (reduction of Body Mass Index) AND (overactive bladder) AND (obese) OR (overweight) OR (obesity) AND (women) OR (female) (Table 1).
| Database | Keywords | Result | Date and Time of Attempt |
|---|---|---|---|
| Cochrane Central Register of Controlled Trials (CENTRAL) | (Body weight reduction): ti,ab,kw OR (weight loss): ti,ab,kw OR (reduced Body Mass Index): ti,ab,kw AND (overactive bladder): ti,ab,kw AND [(obese): ti,ab,kw OR (overweight): ti,ab,kw OR (obesity): ti,ab,kw)] AND [(women): ti,ab,kw OR (female): ti,ab,kw] | 7 | June 11th 2023 (08.05) |
| PubMed/MEDLINE | ("Body weight reduction" OR "weight loss" OR "reduced Body Mass Index") AND ("overactive bladder") AND ("overweight" OR "obese" OR "obesity") AND ("women" OR "female") | 298 | June 11th 2023 (08.01) |
| Embase | ('Body weight reduction': ti,ab,kw OR 'weight loss': ti,ab,kw OR 'reduced Body Mass Index': ti,ab,kw) AND 'overactive bladder': ti,ab,kw AND ('obese': ti,ab,kw OR 'overweight': ti,ab,kw OR 'obesity': ti,ab,kw) AND ('women': ti,ab,kw OR 'female': ti,ab,kw) | 23 | June 11th 2023 (08.10) |
| EBSCO | ("body weight reduction" OR "weight loss" OR "reduced Body Mass Index") AND ("overactive bladder") AND ("overweight" OR "obese" OR "obesity") AND ("women" OR "female") | 23 | June 11th 2023 (08.15) |
| ProQuest | ("Body weight reduction" OR "weight loss" OR "reduced Body Mass Index") AND ("overactive bladder") AND ("overweight" OR "obese" OR "obesity") AND ("women" OR "female") | 371 | June 11th 2023 (08.25) |
| Scopus | TITLE-ABS-KEY [(body weight reduction OR weight loss OR reduced Body Mass Index) AND (overactive bladder) AND (obese OR overweight OR obesity) AND (women OR female)] | 13 | June 11th 2023 (08.30) |
Search Strategy
We utilized EndNote 20 for Windows to compile all articles retrieved from the initial search. Duplicated articles were removed, and each author independently conducted a title and abstract screening process. Full-text articles were assessed for all studies that successfully passed the screening stage.
3.3. Data Extraction and Outcomes of Interest
Each author independently extracted the necessary data from the included studies. This included the name of the first author, year of publication, interventions, follow-up duration, study design, and all pertinent outcomes of interest, along with their respective measurement parameters. For detailed data extraction, please refer to Table 2. The primary outcome of interest in this review is the reduction of OAB symptoms.
| Authors (Ref) | Intervention (n) and Comparators (n) | Follow-up | Design | Outcome Measures | Summary of Findings | |
|---|---|---|---|---|---|---|
| Change in Weight | Improvement of Symptoms | |||||
| Palleschi et al. (19) | I: Weight loss surgery (60) | 5 years | Retrospective cohort | OAB-Q | I: -9.2 ± 0.43 kg/m2 (BMI) | Prevalence UUI: I: -11.7%; C: No change |
| C: Control (60) | Voiding diary | C: 0.1 ± 0.67 kg/m2 (BMI) | Incidence UUI: I: -1.2 ± 0.7 episodes/24h; C: No change | |||
| OAB-Q I: -6.51 ± 5.7; C: No change (P < 0.001) | ||||||
| Whitcomb and Subak (20) | I: Laparoscopic sleeve gastrectomy (176) | 12 months | Prospective cohort | VAS | I: -5.7 ± 0.6 kg/m2 (BMI) | VAS I: -32.2 ± 14.2 (P = 0.002) |
| Cure OAB I: 73% | ||||||
| incidence OAB I: 5% | ||||||
| O’Boyle et al. (16) | I: Laparoscopic gastric bypass (82), laparoscopic sleeve gastrectomy (57), banding (1) | 15 months | Prospective cohort | ICIQ-UI | I: -16 ± 5.2 kg/m2 (BMI) | Cure OAB I: 53% |
| ICIQ-UI SF I: 8 ± 3 | ||||||
| Anglim et al. (21) | I: Laparoscopic gastric bypass (244), sleeve gastrectomy (122) | 12 months | Prospective cohort | NR | I: -18 ± 9 kg/m2 (BMI) | Cure OAB I: 38% |
| Cayci et al. (22) | I: Laparoscopic sleeve gastrectomy (40) | 12 months | Prospective cohort | OAB-Q | I: -18.8 ± 0.94 kg/m2 (BMI) | OAB-Q I: -4.33 ± 1.95 (P < 0.001) |
| Hagovska et al. (6) | I: Programme for Reducing Abdominal Fat (36) | 3 months | Randomised controlled-trial | OAB-Q | I: -1.8 kg/m2 (BMI) | OAB-Q SS: I: -9.91 ± 5.17; C; -1.66 ± 0.51 (P = 0.001) |
| C: Control (34) | Voiding diary | C: No change (BMI) | Incidence UUI: I: -2.05 ± 1.3 episodes/24h; C: No change | |||
| Ait Said et al. (23) | I: Laparoscopic sleeve gastrectomy or bypass (83) | 12 months | Prospective cohort | USP-OAB | I: -13.50 ± 0.3 kg/m2 (BMI) | Prevalence OAB: -36% |
| Waeckel et al. (17) | I: Laparoscopic gastric bypass (53) or sleeve gastrectomy (14) | 6 years | Prospective cohort | USP-OAB | I: -11.7 kg/m2 (BMI) | USP-OAB I: -1, P = 0.01 |
| Kim et al. (24) | I: Roux-en-Y (57) | 12 months | Prospective cohort | OABSS | I: -9.5 ± 3.5 kg/m2 (BMI) | OABSS 1.6 ± 2.3, P < 0.001 |
| Subak et al. (18) | I: Six-month weight loss program (226) | 6 months | Randomised controlled-trial | Voiding diary | I: -7.8 kg (weight) | I: -47.4% (-54.0 to -39.9) (frequency episode) |
| C: Control (112) | C: -1.5 kg (weight) | C: -28.1% (-40.9 to -12.6; P = 0.01) | ||||
Characteristics of the Studies
3.4. Assessment of Methodologic Quality
This review encompasses interventional studies with both trial and retrospective designs. Each author evaluated the quality of the included studies using the risk of bias in non-randomized studies of interventions (ROBINS-I) tool.
4. Results
4.1. Search Results
The details of the study selection process are illustrated in Figure 1. During the initial search, we identified a total of 735 studies. After removing duplicates, 298 unique articles remained. Among these, thirteen studies were relevant to the clinical questions addressed in this review and underwent full-text analysis. However, upon closer examination, three of these studies included male participants, resulting in a total of ten studies included for analysis.
4.2. Included Studies
These ten eligible studies consisted of two clinical trials, seven prospective studies, and one retrospective study. In total, 1,457 patients were included, with 1,049 patients undergoing surgical procedures and 408 patients participating in behavioral weight loss programs. All studies demonstrated an improvement in OAB symptoms among patients undergoing intervention compared to controls, as indicated by a decrease in the OAB-Q score. However, several studies reported using different indicators.
A study by Whitcomb and Subak (20) reported a decrease in the VAS score (-32.2 ± 14.2, P = 0.002). Other studies conducted by Waeckel et al. (17) reported a decrease in the USP-OAB score (-1, P = 0.01), and a study by Kim, et al. reported a low OABSS score (1.6 ± 2.3, P < 0.001) (24). Three prospective cohorts reported the OAB cure rate in the intervention group as follows: (1) 73% in the study by Whitcomb and Subak (20); (2) 53% in the study by O’Boyle et al. (16); and (3) 38% in the study by Anglim, et al. (21). A study by Ait Said, et al. (23) reported a decrease in the prevalence of OAB, reaching 36%. A low incidence of OAB (5%) was also reported in the study by Whitcomb and Subak (20).
Two studies reported a decrease in UUI incidence and prevalence. A study by Palleschi, et al. (19) reported a decrease of 11.7% (UUI prevalence) and 1.2 ± 0.7 episodes/24h (UUI incidence) in the intervention group. Meanwhile, an RCT conducted by Hagovska, et al. (6) reported a decrease of 2.05 ± 1.3 episodes/24h (UUI incidence) in the intervention group. Another RCT conducted by Subak et al. (25), which applied a 6-month weight loss program that included diet, exercise, and behavior modification or a structured education program, showed a decrease in incontinence episodes by 47% (P = 0.01), especially in the frequency of stress-incontinence episodes (P = 0.02). A clinically relevant reduction in the intervention group reached 70% for all types of incontinence. However, the intervention of each study was different. Eight studies applied a surgical intervention, and two studies applied a non-surgical intervention.
4.3. Risk of Bias in Included Studies
The ROBINS-I tool is designed to evaluate the risk of bias (RoB) in the results of non-randomized studies comparing the health effects of two or more interventions. This tool is applicable to quantitative studies that estimate the effectiveness (whether harmful or beneficial) of an intervention, where randomization was not used to allocate participants or clusters of participants to comparison groups (26).
Risk of bias in non-randomized studies of interventions provides a thorough assessment of risk of bias in relation to a hypothetical randomized trial, and “Low risk” of bias corresponds to the risk of bias in a high-quality randomized trial. This opens up the possibility of using the risk of bias assessment, rather than the lack of randomization per se, to determine the degree of downgrading of a study result, and means that results of NRSI and randomized trials could be synthesized if they are assessed to be at similar risks of bias (27).
Risk of bias in non-randomized studies of interventions’s fundamental underlying principles are: (1) The study’s risk of bias is compared against a target randomized controlled trial (RCT), even if this RCT may not be feasible or ethical; (2) the assessment of confounding and selection bias are integral parts of the tool; (3) for a given result for a specific outcome, evidence from an NRS is assessed, addressing a number of domains and then giving an overall rating per outcome for each study (28).
The risk of bias assessment, conducted independently by all authors using the ROBINS-I tool, indicated a low risk of bias in all included studies. Please refer to Table 3 for detailed information on the risk of bias assessment for each study.
| Study ID | Confounding | Selection of Participants | Measurement of Interventions | Deviations from Intended Interventions | Missing Data | Measurement of Outcomes | Selection of the Reported Result | Overall RoB |
|---|---|---|---|---|---|---|---|---|
| Palleschi et al. (19) | Low | Low | Low | Low | Low | Low | Low | Low |
| Whitcomb and Subak (20) | Low | Low | Low | Low | Low | Low | Low | Low |
| O’Boyle et al. (16) | Low | Low | Low | Low | Low | Low | Low | Low |
| Anglim et al. (21) | Low | Low | Low | Low | Low | Low | Low | Low |
| Cayci et al. (22) | Low | Low | Low | Low | Low | Low | Low | Low |
| Hagovska et al. (6) | Low | Low | Low | Low | Low | Low | Low | Low |
| Ait Said et al. (23) | Low | Low | Low | Low | Low | Low | Low | Low |
| Waeckel et al. (17) | Low | Low | Low | Low | Low | Low | Low | Low |
| Kim et al. (24) | Low | Low | Low | Low | Low | Low | Low | Low |
| Subak et al. (18) | Low | Low | Low | Low | Low | Low | Low | Low |
Risk of Bias in Non-randomized Studies of Interventions Assessment of Studies
5. Disscussion
This review has successfully evaluated the impact of weight loss programs on UI symptoms in patients with OAB. It included a total of ten clinical studies, comprising seven prospective cohorts, one retrospective cohort, and two randomized clinical trials. Standard therapy for OAB typically involves lifestyle modifications and the use of antimuscarinic medications. However, there is currently no specific guideline that exclusively recommends weight loss programs as a method to improve UI symptoms in OAB patients (29).
It has been previously explained that the pathogenesis of UI involves increased intra-abdominal pressure, which is typically higher in obese or overweight individuals compared to those of normal weight (7). Overweight OAB patients have also been reported to experience lower health-related quality of life (HRQOL) compared to those of normal weight (30).
Various approaches are employed in weight loss programs. Ultimately, patient comfort and compliance with these programs are crucial factors to consider, as they often need to be sustained over the long term. Weight loss programs that include a low-calorie intake (around 800 kcal/day), regular physical activity, and modifications to eating and exercise habits have been shown to positively impact UI (25).
For example, Hagovska, et al. (6) conducted a study involving 70 OAB women with an average age of 26.4 years and reported a decrease in BMI among patients who underwent a weight loss program by the third month. This reduction in BMI was followed by a decrease in SUI symptoms experienced by the patients. The program included exercises targeting deep abdominal muscles (transversus abdominis, obliquus abdominis internus) and strengthening superficial abdominal muscles (obliquus abdominis externus, rectus abdominis), along with aerobic activity.
A study by Subak, et al. (18) reported that behavioral weight loss programs were effective in decreasing the symptoms of UI. The number of weekly UI episodes decreased by 70% within 6 months after the intervention, with the greatest decrease observed in the frequency of stress-incontinence episodes (18).
Whitcomb and Subak (20) reported a clear dose-response effect of weight on UI, with each 5-unit increase in BMI associated with a 20% - 70% increase in the risk of UI. The maximum effect of weight on UI has an odds ratio of 4 - 5. Additionally, the odds of developing UI over 5 - 10 years increase by approximately 30% - 60% for each 5-unit increase in BMI. There appears to be a stronger association between increasing weight and both prevalent and incident stress incontinence (including mixed incontinence) compared to urge incontinence.
Weight loss studies indicate that both surgical and nonsurgical approaches lead to significant improvements in the prevalence, frequency, and symptoms of UI. Clinicians should recognize the importance of managing BMI through various body weight reduction methods. Uncontrolled increases in BMI, which can lead to metabolic syndrome (MetS), have been shown to heighten the risks and exacerbate the condition of OAB.
Metabolic syndrome can elevate the metabolic rate in bladder tissue, playing a crucial role in mechano-sensory transduction (31). This is facilitated by increased mechanical load, which activates sensory afferents in the bladder, leading to heightened oxidative stress, systemic inflammation, and insulin resistance (31). These factors contribute to chronic pelvic ischemia and urothelial dysfunction. Additionally, a higher BMI may trigger mechanical factors associated with increased intra-abdominal and intravesical pressure (32).
Neuroendocrine processes, triggered by leptin secretion and inflammatory cytokines from visceral adipose tissue, may initiate noradrenergic sympathetic activity and cause urothelial irritation (32). Moreover, obesity can lead to insulin resistance, adversely affecting lipid ratios by reducing HDL cholesterol and increasing triglyceride and LDL cholesterol levels in the bloodstream. These unfavorable cholesterol ratios may contribute to the accumulation of atheromatous deposits in the bladder wall, precipitating bladder wall ischemia, urothelial dysfunction, and an increased risk of OAB (31).
While a gradual decrease in BMI was associated with a reduction in SUI symptoms, the dropout rate during the intervention was high at 26%, partly due to adherence issues with the exercise program (6). Another study with a longer observation period in the lifestyle intervention and weight loss group reported an improvement in UI symptoms. However, at the 18-month follow-up, the difference between the groups in total episodes and SUI was no longer significant due to weight regain (33, 34). It is important to note that lifestyle and dietary interventions alone may not be sufficient to achieve and maintain adequate weight loss in obese women with multiple comorbidities (21).
The advancement of medical technology has facilitated elective surgeries that can significantly reduce patients' weight. One such procedure is bariatric surgery, particularly gastrectomy, which is popular and has proven effective in achieving weight loss (16). However, surgery is usually performed in patients with a BMI > 40 or BMI > 35 with diabetes (35). There are two types of bariatric surgery: Laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux-en-Y gastric bypass (RYGB). In LSG, the lower part of the stomach is removed, resulting in a smaller stomach that limits food intake. After the surgery, patients eat smaller portions due to the reduced stomach size. In contrast, RYGB modifies the transit of food along the gastrointestinal tract by rerouting the passage between the stomach and small intestine. This procedure alters the absorption capacity of food, as the proximal portion of the small intestine is bypassed. Both techniques are effective options that have been proven to aid in significant weight loss (36).
Palleschi, et al. (19) reported that symptoms of OAB were common in the morbidly obese cohort, affecting more women than men. Compared with untreated patients, patients treated with LSG had significantly reduced BMI 180 days postoperatively; this outcome was associated with improvement in OAB symptoms, whereas no change occurred in untreated controls.
Ait Said, et al. (23) confirmed that weight loss after bariatric surgery improves SUI, Urge Incontinence, dysuria, and quality of life.
Anglim, et al. (21) reported postoperative cure rates of 41% for SUI, 38% for OAB, and 48% for mixed incontinence, with 44% of women experiencing complete resolution of their symptoms. The use of postoperative sanitary napkins decreased dramatically from 61% to 36% (P < 0.01). The study also documented a significant improvement in the quality of life of women with OAB, as evidenced by a decrease of 4.8 (5) in the ICIQ-UI SF score, from 9.3 (4.4) preoperatively to 4.5 (5) postoperatively.
Kim, et al. (24) reported that at a 1-year postoperative follow-up after laparoscopic gastric bypass-type bariatric surgery, there were significant improvements in voiding status as assessed by several standard urologic voiding questionnaires/indices.
Waeckel et al. (17) also reported that bariatric surgery seems to be effective at treating SUI and OAB, with long-lasting effects still noted at 6 years.
In a cohort study conducted in 2013 involving 100 Roux-en-Y gastric bypass patients, an average reduction of 10 kg/m² in BMI was observed, with resolution rates of 84% for UI symptoms, 85% for fecal incontinence, and 74% for pelvic organ prolapse (POP) (37).
Furthermore, Ranashinghe, et al. (38) found significant improvement in women assessed using the International Consultation on Incontinence Questionnaire short-form score (P = 0.0008) and quality of life scores (QOL; P < 0.0001). In women, each kilogram of weight loss resulted in an increase of 0.05 in the short-form Incontinence Questionnaire score (P = 0.03).
Other studies have also reported improvements in various comorbidities related to pelvic floor disorders following bariatric surgery. In a study involving 98 women with SUI, OAB, and anal incontinence, symptom resolution was observed in 11 out of 23 (48%) women with SUI, 8 out of 11 (73%) women with OAB, and 4 out of 20 (20%) women with anal incontinence after 12 months of follow-up (39). This suggests that while bariatric interventions can significantly improve some pelvic floor disorders exacerbated by increased intra-abdominal pressure, they may not fully resolve all conditions.
Scozzari, et al. (40) also reported that improvement was limited to UI symptoms, with no significant changes in anorectal function and an increase in flatus incontinence. Despite significantly reducing intra-abdominal pressure, it remains unclear why conditions directly influenced by this pressure do not always improve with bariatric interventions.
Thus, while bariatric procedures lead to weight reduction over time, they also involve other mechanisms that may contribute to the improvement of UI symptoms.
Ranasinghe, et al. (38) and Wesnes (41) suggest that the role of decreased BMI in improving UI symptoms is not solely due to its effect on bladder pressure. O'Boyle, et al. (16) reported that there was no correlation between higher BMI and the rate of weight reduction in terms of the duration and severity of symptoms.
There are other factors that influence the relationship between decreased BMI and bladder pressure. One contributing factor to symptom improvement is the reduction in fluid intake during the early postoperative period. Patients often struggle to meet the recommended minimum fluid intake of 1.5 liters per day in this phase. This aligns with guidelines for fluid intake in behavior change therapy for UI patients.
However, there are no explicit studies assessing the differences in fluid intake and output in patients undergoing bariatric interventions.
In addition to the natural fluid restriction that occurs after bariatric surgery, there is also a decrease in the amount of adipose tissue that produces leptin in the bloodstream. Leptin influences the autonomic nervous system, particularly the noradrenergic sympathetic nerves, which play a role in the pathophysiology of incontinence (42).
Despite the satisfactory results, bariatric surgery is associated with several complications, including persistent nausea and vomiting, intolerance to solid food, and severe dumping syndrome (43). A multicenter study in India involving over 10,000 patients reported a complication rate of 3.13% for bariatric surgery (n = 363), which is consistent with findings reported by Melissas, et al. of 2.1% to 3% (44, 45). The mortality rate associated with this procedure ranges from 0.16% to 0.22% (46).
Daigle, et al. (47) identified bleeding and leakage as the complications with the most significant overall impact, leading to organ failure, reoperation, and admission to the intensive care unit following bariatric surgery. Therefore, the decision to proceed with bariatric surgery should consider the patient's comprehensive risk of morbidity and mortality.
This review demonstrates that effective weight loss strategies often involve a combination of dietary changes, exercise, and, in some cases, bariatric surgery. Programs integrating low-calorie intake and physical activity have shown improvements in UI symptoms. Bariatric surgery, particularly Laparoscopic Sleeve Gastrectomy and Roux-en-Y Gastric Bypass, has also demonstrated substantial benefits in reducing UI symptoms and improving quality of life. However, invasive procedures carry several complications, and not all obese patients are suitable candidates for these interventions.
Unfortunately, the precise mechanisms underlying the association between obesity and incontinence remain unclear, and additional neurophysiological and urodynamic studies are needed to better define the obesity–UI relationship.
It is also important to acknowledge several limitations of the included studies. This review encompasses studies with unstandardized outcome measures and varying approaches to weight loss programs. Current data are limited by both short-term follow-up and unexplained heterogeneity among studies. Finally, it is crucial to note that some of the clinical studies mentioned are retrospective studies based on prospective cohorts, which introduce inherent biases in the study design.
5.1. Conclusions
Evidence from multiple clinical studies supports that weight loss programs are effective in reducing UI symptoms in patients with OAB, whether through surgical or non-surgical procedures. While lifestyle interventions focusing on calorie reduction and physical activity have shown promising results, bariatric procedures such as Laparoscopic Sleeve Gastrectomy and Roux-en-Y Gastric Bypass provide significant weight reduction and symptom relief for morbidly obese patients.
Clinicians should consider individualized approaches to weight management, taking into account the potential complications of surgical interventions and the importance of maintaining long-term weight loss for optimal patient outcomes.
Recommendations for future research should aim to better understand the underlying mechanisms linking obesity and UI; use standardized outcome measures to facilitate comparisons between studies; and include literature with RCT designs to reduce bias and enhance the validity of results. Additionally, it is crucial to investigate factors that may explain the heterogeneity observed among existing studies.