1. Background
Interstitial cystitis (IC) or painful bladder syndrome (PBS) is a bladder problem with an unknown cause. The female-to-male ratio of cystitis is 9:1 in surveys of affected cases in the United States (1). Painful bladder syndrome is defined by the International Continence Society (ICS) as "pain of suprapubic pertaining to bladder filling, along with other clinical manifestations including increased nighttime and daytime frequency, in the absence of obvious pathology such as urinary infection" (2). The diagnosis of this condition is based on accurate evaluation of urinalysis, symptoms, physical examination, cystoscopy with biopsy, pelvic ultrasound, and urine culture to differentiate PBS/IC from other causes of these symptoms (3, 4).
Allergic rhinitis and asthma affect more than 300 million individuals globally, with 10 - 30 percent of adults and over 40 percent of pediatric cases being impacted (5). This condition could potentially be related to cystitis. Montelukast, an antagonist of leukotriene (LT) receptors, was approved by the FDA in 1998 and 2002 for the treatment of asthma and allergic rhinitis, respectively (6). Recently, asthma treatment guidelines highlighting LTRAs as alternative controller methods have noted a lack of evidence for their safety or efficacy. Similarly, allergic rhinitis guidelines restrict this drug to cases where alternative methods are unsuccessful (7).
Various medicinal treatments have been evaluated and approved for treating this disorder. Among these drugs, pentosan, Bacillus Calmette-Guerin, tricyclic antidepressants, heparin, and dimethyl sulfoxide have been studied for this condition (7). Montelukast, a medication that blocks LTD4 receptors, is commonly used in cases of asthma and allergic rhinitis in clinical practice. Previous research has observed LTD4 receptors in human bladder detrusor myocytes and found that this drug exerts an anti-inflammatory effect by inhibiting LT receptors in the bladder (8, 9). Based on these findings, montelukast is hypothesized to be a potential treatment for IC, though it has not been adequately studied in this context.
As stated, IC is a common condition; however, there is insufficient research on its treatment and etiology (7). Studies have noted that inflammation plays a significant role in IC pathogenesis. The presence of LT receptors on detrusor cells and elevated levels of urinary LT-D4 in IC underscore the inflammatory component of the condition (10). Additionally, an increased concentration of mast cells in detrusor cells has been reported (9, 11). However, only a few studies have evaluated the effects of anti-inflammatory agents.
2. Objectives
Therefore, the present study investigates the potential effectiveness of montelukast as a treatment for cystitis in asthmatic pediatric cases.
3. Methods
3.1. Study Setting and Population
The present study was a clinical trial conducted at Amir Kabir Hospital, Arak, Iran, involving pediatric cases with cystitis. The sample size for this study was calculated using the results of a similar study (12) and the STATA 11 software, with a confidence level of 95% and a power of 80%. Each group included 28 cases, resulting in a total of 56 pediatric cases evaluated: Twenty eight in the montelukast group and 28 in the control group.
3.2. Inclusion and Exclusion Criteria
3.2.1. Inclusion Criteria
- Cystitis in asthmatic pediatric cases.
- Definitive diagnosis of cystitis by a pediatric urologist.
- Rule out other etiologies of cystitis.
- 5 to 15 years of age.
- History of allergies.
- And informed consent to participate in the study.
3.2.2. Exclusion Criteria
- Allergic reactions to drugs.
- Contraindications of montelukast.
- Failure to take medication correctly by the patient.
- Taken antibiotics in the last 10 days.
- And the unwillingness of the patient or parents to continue participating in the study.
3.3. Measurements
This clinical trial was conducted on 56 cases aged 5 to 15 years. These cases, after receiving a definitive diagnosis of cystitis based on inclusion criteria and obtaining informed consent from parents, were enrolled in the study. Using a simple randomization method, the patients were divided into two equal groups: The montelukast group and the control group.
All cases in both groups received the standard treatment for cystitis, consisting of cefixime at a dose of 8 mg/kg daily for 10 days. Additionally, in the first group, montelukast (Sobhan Daro, Iran) was administered as 5 mg orally daily for 10 days. In the control group, only the standard treatment for cystitis with cefixime was prescribed.
The most common symptoms of the disease, including urinary frequency, urgency, suprapubic pain, and hematuria, were explained to the parents. They were then asked to report the presence or absence of these symptoms during the 10 days of cystitis treatment. The acquired data were entered into SPSS19 statistical software, and statistical analyses were performed using the t-test and Fisher’s exact test.
3.4. Statistical Analysis
The acquired data were statistically evaluated using the SPSS program. Qualitative data were presented as percentages and frequencies, while quantitative data were presented as means and standard deviations (SDs). In inferential statistics, Fisher's exact test, chi-square test, independent sample t-test, their non-parametric equivalents, and covariance analysis were used to test the hypotheses.
4. Results
4.1. Age and Gender of Evaluated Cases
Of the 56 evaluated cases, the mean and SD of age in the montelukast group were 7.0 ± 2.24 years, and in the control group, they were 6.5 ± 2.78 years. Additionally, the male-to-female ratio in the montelukast group was 12/16 (42.9%/57.1%), and in the control group, it was 10/18 (35.7%/64.3%) (Table 1).
| Variables | Groups | Total | |
|---|---|---|---|
| Montelukast | Control | ||
| Age | 7.0 ± 2.24 | 6.5 ± 2.78 | 6.75 ± 2.51 |
| Gender (M/F) | 12/16 (42.9/57.1) | 10/18 (35.7/64.3) | 22/34 (39.3/60.7) |
4.2. Recovery Time of Clinical Manifestations
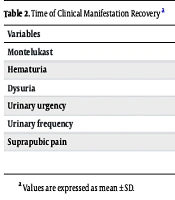
Based on the evaluation of recovery time for clinical manifestations in the two groups, hematuria recovery time in the montelukast group was 1.91 ± 0.42 days, compared to 1.89 ± 0.5 days in the control group. Dysuria recovery times in the montelukast and control groups were 2.96 ± 2.60 and 3.21 ± 3.30 days, respectively (P = 0.174). Additionally, urgency recovery times in the montelukast and control groups were 1.39 ± 2.39 and 2.71 ± 3.84 days, respectively. Recovery times for urinary frequency in the montelukast and control groups were 4.17 ± 3.93 and 3.85 ± 4.08 days, respectively. Suprapubic pain recovery time in the montelukast group was 1.42 ± 2.75 days compared to 2.00 ± 2.99 days in the control group. Based on statistical evaluation, urgency showed a significant difference between the two groups (P = 0.005). However, other clinical manifestations did not show significant differences, including hematuria (P = 0.956), dysuria (P = 0.174), urinary frequency (P = 0.576), and suprapubic pain (P = 0.706) (Table 2).
| Variables | Groups | P-Value | |
|---|---|---|---|
| Montelukast | Control | ||
| Hematuria | 1.91 ± 0.42 | 1.89 ± 0.5 | 0.956 |
| Dysuria | 2.96 ± 2.60 | 3.21 ± 3.30 | 0.174 |
| Urinary urgency | 1.39 ± 2.39 | 2.71 ± 3.84 | 0.005 |
| Urinary frequency | 4.17 ± 3.93 | 3.85 ± 4.08 | 0.576 |
| Suprapubic pain | 1.42 ± 2.75 | 2.00 ± 2.99 | 0.706 |
a Values are expressed as mean ± SD.
5. Discussion
Interstitial cystitis, as a chronic condition, presents with clinical manifestations such as frequency, urinary urgency, suprapubic pain, and hematuria. Although this disease is common, there is insufficient research regarding its treatment and etiology. Studies have indicated that inflammation plays a significant role in the pathogenesis of IC. The presence of LT receptors on detrusor cells and elevated levels of urinary LT-D4 in IC demonstrate an inflammatory role in the disease. Additionally, an increased concentration of mast cells in detrusor cells has been noted. However, few studies have evaluated the effects of anti-inflammatory agents, leading to the evaluation of montelukast as an effective drug for cystitis in asthmatic pediatric cases in the present study.
Based on statistical evaluation, urgency showed a significant difference between the two groups (P = 0.005). However, other clinical manifestations, including hematuria (P = 0.956), dysuria (P = 0.174), urinary frequency (P = 0.576), and suprapubic pain (P = 0.706), did not show significant differences.
In two case reports by Wajih Ullah et al. and Traut et al., a 26-year-old female and a 64-year-old male diagnosed with PBS/IC were treated with montelukast (13, 14). However, in the present study, montelukast had a significant effect only on urinary urgency. Additionally, Gunizi et al. observed in a study on rats that inflammatory mediators were significantly decreased in the montelukast group in cases with IC (8).
Regarding the possible mechanism, previous studies have mentioned an increased number of mast cells in many instances (15). In IC, complete or partial mast cell degranulation can be detected in the bladder muscle, submucosa, and lamina propria. Fall et al. reported that a cutoff of twenty mast cells/mm² in bladder muscle exhibited diagnostic sensitivity of 95% and diagnostic specificity of 88% for IC (16). In non-ulcer IC cases, mast cells in bladder muscle showed large SDs due to heterogeneous research groups and differences in methods (10). Although the etiology of mast cell enhancement in IC is unclear, certain cytokines, such as stem cell factor (SCF) and nerve growth factor (NGF), are recognized as mast cell stimulators (15). Nociceptive molecules, vasoactive agents, and pro-inflammatory substances released from mast cells are believed to cause sensory neuronal hyperreactivity and neuropathic pain in IC (17).
Mast cells function through intermediaries such as pro-inflammatory and vasoactive mediators, synthesized and stored in granules (15, 18). Preformed molecules include kinins, histamine, proteases, serotonin, and TNF, while de novo synthesized molecules include platelet-activated factor (PAF), LT, various interleukins (IL), vascular endothelial growth factor (VEGF), prostaglandins, and nitric oxide (NO). These cytokines and amines, released without mast cell degranulation, serve as markers (19). Thus, the potential mechanism of montelukast's effect on IC can be explained through its influence on these inflammatory pathways.
Leukotrienes, as a bioactive group, are produced from the metabolism of arachidonic acid (20). LTC4, LTE4, and LTD4, known as slow-acting anaphylaxis agents, play an important inflammatory role. Additionally, LTA4 serves as a precursor in LT synthesis and is metabolized to form LTC4, which is subsequently converted to LTD4 in the extracellular space (11). In some studies and literature, it has been observed that symptom frequency and pain complaints decrease with montelukast in IC, which is known to influence mast cell function and pathophysiology. Thus, this agent has been identified as a potential treatment alternative (15). Despite both basic and clinical studies on the treatment of IC, a condition with increasing incidence, a highly effective treatment has not yet been established. However, the presence of LTD4 receptors in the human detrusor muscle has been detected in research (9).
TNF alpha, an inflammatory agent, impairs wound healing when present in chronic doses (21). TNFα, released from activated macrophages, leads to an increase in free oxygen radicals and the expression of adhesion factors in the vascular endothelium (22). TNF alpha is a major component of the soluble factors released by mast cells that mediate the urothelial response (23). Another cell culture study demonstrated that mast cells and TNFα contribute to apoptosis in IC (24). Despite clinical and basic research on IC, an escalating health problem, an effective standard treatment has not yet been determined.
The literature includes evidence of LTD4 receptors in human detrusor myocytes (9). LTD4, a pro-inflammatory mediator produced by mast cells in the detrusor muscles, induces a spasmogenic effect on the bladder and is responsible for the symptoms and pain associated with IC. Montelukast, widely used in the treatment of allergies, blocks LTD4 receptors and has been shown in previous studies to exert an anti-inflammatory effect through this pathway (8, 25). Based on this, montelukast, as a LT-D4 antagonist, can be considered a potential effective agent for IC treatment. However, further systematic review studies are needed to confirm its efficacy in this context.
5.1. Conclusions
According to the results of the present study and in comparison to the findings of other studies, the benefit of montelukast on urgency was clinically greater than that of the control group. However, montelukast did not show significant efficacy on hematuria, urinary frequency, or suprapubic pain. Therefore, in asthmatic pediatric cases, a 10-day treatment of cystitis with montelukast can reduce the duration of urinary urgency in children, but it has no effect on urinary burning, urinary frequency, or suprapubic pain.
It is recommended that future studies include a larger sample size and investigate the effects of different doses of montelukast. Additionally, further research should explore the effects of other anti-inflammatory drugs on cystitis and compare their efficacy with that of montelukast.
5.2. Limitations
One of the limitations of this evaluation was the lack of cooperation from parents, which was mitigated to some extent by explaining the benefits of their participation. Another limitation was the small sample size, which was partially addressed by extending the sampling period.