1. Background
Infertility is characterized as a couple’s inability to achieve pregnancy after one year of consistent, unprotected sexual activity (1, 2). Overall, approximately 15% of couples experience infertility at some point in their married life, with male factors contributing to less than 50% of cases (3).
It is estimated that around 20.2% of Iranian couples are infertile, which is significantly higher than the universal rate of 12% - 15%; 70% of infertility cases were reported to be related to male factors. Several reasons have been proposed for male infertility, including anti-sperm antibody production, defective delivery of sperm, obstruction of the seminal tract, etc.; among these, 40% - 90% of male infertility cases are said to be related to impaired spermatogenesis, which is a significant rate (2). Semen analysis of men suffering from impaired spermatogenesis shows abnormal semen parameters manifested as azoospermia, oligozoospermia, teratozoospermia, and asthenozoospermia (4). The cause of male infertility is idiopathic and could be congenital or acquired. Among the different reasons for male infertility, elevated levels of reactive oxygen species (ROS) and oxidative stress (OS) in semen and sperm are involved in 30% to 80% of cases (5, 6).
Increased ROS levels reduce sperm fertilizing capacity by impairing axonemal phosphorylation, causing DNA damage, decreased viability, motility, and lipid peroxidation (LPO). Furthermore, antioxidant compounds have been shown to protect tissues and cells against the detrimental impacts of ROS, further improving male fertility and sperm quality (7, 8). They also lead to statistically significant increases in both live birth rates and pregnancy rates (9).
Oral sperm quality enhancement medications can be among the initial treatment options in the therapeutic process, and statistics indicate that patients typically have a strong preference for initiating treatment with these due to their ease of administration. BlooMax, a recently introduced oral tablet in the Iranian pharmaceutical-therapeutic market, contains a range of vitamins (B6, B9, B12, C, D3, and E), amino acids (L-cysteine, L-arginine, and L-carnitine), and antioxidants (selenium, coenzyme Q10 (Co-Q10), zinc as zinc gluconate, beta-carotene, and pine bark extract). Various clinical studies and meta-analyses have separately examined the effects of some vitamins, antioxidants, or amino acids on male fertility, and they have positively influenced sperm count and quality, as well as improved male sexual health (6, 10-15).
2. Objectives
So far, to the best of our knowledge, no study has examined the effect of the supplement BlooMax on male infertility. On the other hand, given the high prevalence of male infertility and its consequences for public health, such as mental discomfort, social stigma, economic stress, and marital separation (16), and the limited number of research studies on the effect of oral vitamins on semen parameters in Iranian infertile men, we investigated the effects of BlooMax administration on sperm quality in infertile men in Bandar Abbas.
3. Methods
This double-blind (patient and investigator) clinical trial was conducted on non-obstructive azoospermia infertile men (with a history of more than one year of infertility without using contraceptive methods) who were referred to urology clinics and infertility centers in Bandar Abbas. All research stages were approved by the Ethics Committee of Islamic Azad University, Qeshm Branch (IR.IAU.BA.REC.1403.001) and registered on the Iranian Registry of Clinical Trials (IRCT20241031063553N1). Following the acquisition of ethical approvals and the application of inclusion and exclusion criteria, patients were randomly assigned to the intervention (BlooMax) or control (placebo) groups.
3.1. Criteria
3.1.1. Inclusion Criteria
(1) Age 18 to 45 years; (2) being married (based on birth certificate); (3) more than one year of infertility [as defining by Eisenberg (2)]; (4) no history of genitourinary or pelvic surgery, such as vasectomy, varicocele, hernia repair, etc. (by asking the patient).
3.1.2. Exclusion Criteria
(1) History of angioplasty; (2) history of various malignancies or chronic physical/mental illnesses, including cardiac, renal, immunological, or hepatic illnesses, and acquired immunodeficiency syndrome (AIDS); (3) use of vitamin supplements in the past two months; (4) regular sleep at night; (5) controlled underlying diabetes and hypertension; (6) use of cigarettes, alcohol, or narcotics.
3.2. Sample Size and Calculation Method
Based on Sadaghiani et al.’s study (17), considering a 90% effect of antioxidants in improving sperm quality, a type I error of 5% (95% confidence level), a power of 80% (type II error of 0.2), and with the application of a population correction factor, approximately 50 samples per group, and a total of 100 patients overall, were estimated.
In this study, 100 infertile male cases were enrolled. Fifty of them were assigned to the control (placebo) group, and the other 50 received the BlooMax medication in the intervention group.
3.3. Sampling Method
The project manager used a randomized block method to assign eligible patients between experimental and control groups. Random allocation using blocks of 2 started by throwing 50 regular hexagons, then generating a sequence of 50 blocks of 2 from the following blocks: AB-BA-BB-AA. Patients were then randomly divided into two intervention groups using the four-block method. Group A received BlooMax, and group B received a placebo. Randomization was achieved by using dice to select the first block, and patients were selected according to their position in the initial blocks to the end. The allocation ratio was 1:1.
3.4. Data Collection
All infertile men received standard treatment. Subsequent to initial evaluations by a specialist and obtaining the results, both groups commenced the administration of BlooMax (three tablets daily in a single dose with water, before meals, for three months) in the intervention group and a placebo (sugar pills with the same shape and size) in the control group, according to a pre-determined schedule. They continued consumption regularly as pre-determined and explained, and also maintained regular sexual intercourse (three times per week).
During this period, before the completion of the pharmacotherapy course and subsequently at several intervals, semen samples were collected from the patients for sperm quality analysis and comparison and sent to a pre-determined laboratory. For this purpose, participants were asked to observe a 3 - 5 day period of sexual abstinence and repeat their semen assessment. Sperm total motility, morphology, concentration, progressive motility, and the Sperm Motility Index (SMI) were evaluated. The data and results were meticulously recorded, documented, and compared.
3.5. Semen Analysis
To evaluate sperm parameters, the World Health Organization (WHO) 2010 instructions were followed, and a sperm quality analyzer V (SQAV) and a light microscope (for morphology assessment) were utilized. In this study, all sperm parameters, except for morphology, were evaluated by the SQAV. Various results regarding accuracy and precision were reported when evaluating the accuracy of computer-assisted semen analyzers (CASA and SQAV) and the conventional manual method.
3.6. Data Analysis
All experiments were conducted in triplicate, with the data expressed as mean ± standard deviation (SD) along with frequency percentages. Data analysis was carried out using SPSS 19 software. The normal distribution of the data was assessed by the Shapiro-Wilk test. Comparisons between the groups were made using ANOVA or t-test, with a P-value of less than 0.05 deemed significant.
4. Results
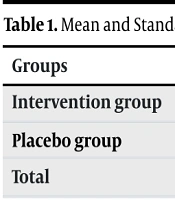
In this study, 100 participants were divided into two groups of 50, and all completed the study. The infertile men’s mean age was 32.61 ± 4.53 years. Furthermore, as shown in Table 1, no significant difference was detected in the mean age between the placebo and intervention groups, indicating successful matching.
| Groups | Mean ± Standard Deviation | P-Value |
|---|---|---|
| Intervention group | 32.24 ± 4.35 | 0.418 |
| Placebo group | 32.98 ± 4.73 | |
| Total | 32.61 ± 4.53 |
Mean and Standard Deviation of Age in the Case and Control Groups
In this study, all quantitative variables were assessed for normality using the Shapiro-Wilk test, and the normality of their statistical distribution was confirmed. Consequently, parametric tests were utilized to compare these variables between the control and intervention groups. Table 2 presents the sperm quality parameters, including ejaculate volume, pH, white blood cell (WBC) count (indicating infection) (P = 0.089), progressive motility (P = 0.053), non-progressive motility (P = 0.078), immotile sperm (P = 0.052), and abnormal (P = 0.180) and normal morphology (P = 0.180) between the intervention and control groups of infertile men. No significant difference was detected in the aforementioned parameters between the two groups before the intervention.
| Variables | Before Intervention Case Group | Before Intervention Placebo Group | 95% CI (Lower - Upper) | P-Value |
|---|---|---|---|---|
| Abstinence | 2.32 ± 0.58 | 2.52 ± 0.07 | -0.45 - 0.05 | 0.118 |
| Liquefaction | 30 ± 0.01 | 30 ± 0.01 | - | - |
| Volume | 3.17 ± 1.35 | 3.49 ± 0.94 | -0.42 - 0.42 | 0.993 |
| PH | 7.31 ± 0.07 | 7.28 ± 0.05 | -0.011 - 0.031 | 0.362 |
| Round cell | 2.44 ± 1.28 | 2.40 ± 1.01 | -0.2007 - 0.560 | 0.350 |
| WBC | 1.84 ± 1.37 | 1.54 ± 1.14 | -0.9694 - (-0.190) | 0.089 |
| Vitality | 61.48 ± 2.17 | 0 | - | - |
| Concentration before | 23.61 ± 19.43 | 22.74 ± 14.58 | 2.368 - 17.111 | 0.07 |
| Progressive | 21.98 ± 15.97 | 25.78 ± 13.36 | -0.0728 - 13.032 | 0.053 |
| Non-progressive | 36.11 ± 14.96 | 37.14 ± 19.09 | -22.766 - (-9.433) | 0.078 |
| Immotil | 41.72 ± 20.53 | 38.08 ± 14.22 | 1.266 - 15.613 | 0.052 |
| Normal morphology | 2.22 ± 1.29 | 2.70 ± 1.11 | -0.243 - 1.283 | 0.180 |
| Abnormal morphology | 97.78 ± 1.29 | 97.31 ± 1.11 | -1.283 - 0.243 | 0.180 |
| ERC | 6.20 ± 2.98 | 6.34 ± 3.13 | 1.678 - 4.401 | 0.094 |
| Head | 89.26 ± 18.61 | 85.60 ± 10.45 | -9.318 - 2,998 | 0.311 |
| Neck | 58.12 ± 24.66 | 51.96 ± 14.91 | 9.412 - 25.587 | 0.06 |
| Tail | 27.32 ± 15.64 | 21.84 ± 8.91 | -6.296 - 3.896 | 0.641 |
Comparison of Mean and Standard Deviation of Sperm Quality Variables in the Case and Control Groups Before Intervention
According to Table 3 and the t-test analysis, the assessment of sperm quality parameters revealed a significant elevation in the total number of sperm cells per ejaculate in the intervention group following BlooMax administration, rising from 23 million to 30 million (P = 0.001). Furthermore, there was a significant increase in progressively motile sperm and a significant reduction in both non-progressively motile and immotile sperm (P = 0.001 for both). The table also demonstrates a significant elevation in the number of sperm with normal morphology, rising from 2.2 million to 3.2 million. Ultimately, it was found that BlooMax administration resulted in a reduction in morphological defects, including head, neck, and tail abnormalities, in the infertile men of the intervention group (P = 0.001 for all).
| Variables | Case Groups | P-Value | |
|---|---|---|---|
| Before Intervention | After Intervention | ||
| Abstinence | 2.32 ± 0.58 | 2.33 ± 0.55 | 1 |
| Liquefaction | 30 ± 0.01 | 30 ± 0.01 | 1 |
| Volume | 3.17 ± 1.35 | 3.49 ± 1.18 | 0.242 |
| PH | 7.31 ± 0.07 | 7.29 ± 0.05 | 0.264 |
| Round cell | 2.44 ± 1.28 | 2.58 ± 0.91 | 0.461 |
| WBC | 1.84 ± 1.37 | 1.96 ± 0.78 | 0.604 |
| Concentration before | 23.61 ± 19.43 | 30.48 ± 21.84 | 0.001 |
| Progressive | 21.98 ± 15.97 | 32.26 ± 19.14 | 0.001 |
| Non-progressive | 36.11 ± 14.96 | 31.04 ± 14.12 | 0.001 |
| Immotil | 41.72 ± 20.53 | 36.52 ± 21.24 | 0.001 |
| Normal morphology | 2.22 ± 1.29 | 3.22 ± 2.48 | 0.001 |
| Abnormal morphology | 97.78 ± 1.29 | 96.78 ± 2.48 | 0.001 |
| ERC | 6.20 ± 2.98 | 8.38 ± 3.70 | 0.001 |
| Head | 89.26 ± 18.61 | 82.44 ± 19.29 | 0.001 |
| Neck | 58.12 ± 24.66 | 49.46 ± 24.65 | 0.001 |
| Tail | 27.32 ± 15.64 | 20.64 ± 15.82 | 0.001 |
Comparison of Mean and Standard Deviation of Sperm Quality Variables in the Case Group Before and After Intervention
In men with low-quality semen, dietary antioxidant supplements have shown promise in enhancing overall sperm quality by mitigating OS-related sperm damage and improving hormone production, sperm morphology, motility, and concentration.
5. Discussion
Recently, the impact of OS on male infertility etiology, as well as the effect of oral antioxidant supplements on improving semen quality in infertile men, has been considered (18). In our study, the assessment of sperm quality parameters revealed a marked elevation in the total sperm count per ejaculate in the intervention group following BlooMax administration. Progressively motile sperm significantly increased, while non-progressive and immotile sperm counts significantly decreased. Additionally, the administration of this medication resulted in an elevated number of normal sperms and decreased morphological defects, including head, neck, and tail abnormalities, in infertile men compared to the controls.
Multi-antioxidant supplements are currently regarded as an effective treatment for male infertility. The synergistic effect of multi-antioxidants has made them of interest to researchers. A study in this regard is Sadaghiani et al.’s study, which aimed to assess the impact of vitamin supplement administration on changes in infertility parameters of male smokers (50 infertile oligozoospermic and asthenozoospermic men). A comparison of pretest and posttest results revealed a statistically significant elevation in all sperm parameters, such as progressive motility, volume, count, morphology, and motility, after the intervention. Moreover, significant improvements were observed in semen pH and concentration (17).
Another study assessed the effect of antioxidant treatment on natural pregnancy and the quality of semen parameters in the partners of males experiencing persistent oligozoospermia (5 to 20 million/mL) six months following retrograde embolization. In their study, 20 males experiencing varicocele underwent antioxidant treatment with vitamins and minerals (vitamins C, E, and A, B12, manganese, thiamine, magnesium, biotin, riboflavin, iron, copper, and zinc) and N-acetylcysteine (NAC). Following the treatment, the treated groups showed a significant increase in sperm count. A significant correlation was also found between multi-antioxidant supplement administration and semen morphology and motility (19).
In a similar vein, Abad et al. evaluated the effect of oral antioxidant treatment on sperm DNA fragmentation dynamics in 20 infertile patients experiencing asthenoteratozoospermia. All participants were treated with 10 mg of vitamin E, 20 mg of Co-Q10, 1500 mg of L-carnitine, 60 mg of vitamin C, 200 μg of folic acid, 10 mg of zinc, 1 μg of vitamin B12, and 50 μg of selenium over a 3-month period. The proportion of sperm DNA fragmentation significantly reduced, and semen assessment showed a significant elevation in motility, morphology, concentration, and viability. Furthermore, DNA integrity significantly improved at all incubation times. Antioxidant treatment improved sperm quality regarding key semen parameters and baseline DNA damage and could maintain DNA integrity. Hence, antioxidant agents can be beneficial in novel medical therapies (20).
The aforementioned studies align with and corroborate the findings of our study. BlooMax is a dietary supplement formulated to enhance male fertility, containing a group of vitamins, minerals, amino acids, and antioxidants, including B6-B12 vitamins, vitamins C, D, and E, Co-Q10, folic acid, selenium, L-arginine, L-carnitine, L-cysteine, zinc, beta-carotene, etc. The antioxidants in BlooMax reduce and neutralize free radicals, increase antioxidant enzyme activity, or act as a source of energy for sperm.
Research has also assessed the effects of antioxidants and vitamins, either individually or in combination, on enhancing male sexual function and sperm quality. For instance, in Ciftci et al.’s study exploring the impact of NAC on semen factors and the antioxidant/oxidative status in male infertility, 120 cases were assigned to two groups: Placebo and intervention. Their study results revealed that although NAC significantly improved semen volume, motility, and viscosity, it caused no significant differences in sperm count or morphology between the two groups (21).
In another study, Raigani et al. assessed the impact of zinc sulfate and folic acid supplements on improving sperm function in infertile males with oligoasthenoteratozoospermia (OAT). In their study, 83 men with OAT participated in a randomized, double-blind, clinical trial intervention for 16 weeks and daily received zinc sulfate (220 mg/day) and folic acid (5 mg/day) or placebo. Sperm chromatin integrity (%) showed a significant increase in infertile males treated with zinc sulfate alone. Nonetheless, such an improvement in sperm quality was non-significant following adjustment for the placebo impact. Folic acid and zinc sulfate supplements could not improve sperm quality in infertile males with sperm parameters highly affected by OAT (22).
Additionally, Lahimer et al. conducted a study to explore the impact of oral antioxidant therapy (Fertilis), containing L-carnitine and certain micronutrients, on improving sperm DNA integrity, common sperm parameters, and outcomes of in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI). The study assessed 263 participants undergoing IVF/ICSI, and the subjects were randomly allocated to the placebo and treatment groups. One hundred thirty-one cases underwent antioxidant treatment, and 132 were treated with the placebo over three months. Sperm motility and clinical pregnancy rate significantly increased. However, conventional sperm parameters (vitality, count, and volume) or DNA sperm Decondensation Index (SDI) showed no significant changes (14).
The reasons for the ineffectiveness of prescribed supplements in infertile men, as reported in the studies, appear to be attributed to the short duration of supplement intake or the administration of single-agent supplements.
Male infertility is significantly influenced by OS. Therefore, proper sperm production, function, and viability necessitate a balance between OS and antioxidants. In men with low-quality semen, dietary antioxidant supplements have shown potential in enhancing overall sperm quality by reducing OS-related sperm damage and improving hormone production, sperm concentration, motility, and morphology.
5.1. Conclusions
Comparison of sperm parameters before and after the intervention demonstrated that the consumption of supplements containing vitamins and antioxidants (BlooMax) significantly enhanced sperm total motility, volume, morphology, count, and progressive motility. This improvement in sperm quality can lead to reduced infertility due to sperm defects and may serve as an adjuvant treatment in infertile men.
5.2. Limitations
The limitations of the present study included a limited sample size, which necessitates further investigation with larger-scale studies in the future. Additionally, other parameters, such as DNA damage, could not be assessed, which requires evaluation in future studies with increased sample sizes.
5.3. Suggestions
Given the novelty of studies examining the effects of infertility drugs and the small sample size in this study, a larger trial with a greater focus on the side effects of this medication would be preferable to consolidate the results of this study.