1. Introduction
1.1. Epidemiology and Clinical Burden of Non-obstructive Azoospermia
Male infertility accounts for approximately 50% of all infertility problems, as 50% of all infertile couples present with abnormal semen parameters (1). Among all male infertility cases, azoospermia is one of the most challenging to deal with. Azoospermia accounts for about 11.2% of all male infertile patients (1). It is further classified as obstructive azoospermia (OA) and non-obstructive azoospermia (NOA) (1).
1.2. Overview of Current Sperm Retrieval Techniques and Their Limitations
Managing OA is relatively simpler compared to NOA, as the latter involves focal spermatogenesis instead of generalized spermatogenesis in a normal male. The focal nature of the disease has made it very challenging to find sperm during conventional sperm extraction procedures (Table 1) (2).
| Sperm Retrieval Technique | Sperm Retrieval Rate (%) |
|---|---|
| TESA | 11 - 47.3 |
| TESE | 16,7 - 59 |
| MicroTESE | 42 - 63 |
Abbreviations: TESA, testicular sperm aspiration; TESE, testicular sperm extraction; MicroTESE, microsurgical testicular sperm extraction.
1.3. Conventional Sperm Retrieval Success
Sperm retrieval techniques can be categorized into two main groups: Epididymal aspiration and testicular sperm retrieval. Epididymal aspiration includes percutaneous epididymal sperm aspiration (PESA) and microsurgical epididymal sperm aspiration (MESA), while testicular sperm retrieval involves testicular sperm aspiration (TESA), testicular sperm extraction (TESE), and microsurgical testicular sperm extraction (microTESE). Epididymal aspiration is primarily used for OA, while testicular sperm retrieval is mainly used for NOA. The sperm retrieval rates for these techniques are summarized in Table 1 (12).
Conventional sperm extraction methods often fail when performed in areas without spermatogenesis, as the procedures are conducted blindly. Additionally, testicular sperm retrieval can cause permanent structural damage, leading to lower testosterone levels and potential hypogonadism. A recent systematic review has demonstrated that microTESE provides the highest sperm retrieval success rate, being 1.5 times more likely to retrieve sperm compared to conventional TESE. Furthermore, conventional TESE is twice as likely to yield successful sperm retrieval compared to TESA (13).
In 2019, Vieira et al. introduced open testicular mapping (OTEM), a novel sperm retrieval technique. This method involves a median scrotal incision and multiple punctures (up to six) through the tunica albuginea using a 19G needle. Testicular compression is applied to allow the protrusion of tubules through the punctures. The study reported that OTEM achieved a sperm retrieval rate of 54%, comparable to TESE and microTESE, and allows for multiple samples to be taken without excessive testicular dissection (14).
1.4. Rationale for the Use of Testicular Mapping Biopsy
1.4.1. Testicular Mapping Biopsy
Testicular mapping biopsy was first introduced by Turek et al. in 1997 (15). This procedure offers the benefits of minimal invasiveness, cost-effectiveness, and minimized testicular injury while providing valuable information on focal spermatogenesis areas. While this technique has numerous benefits and advantages, it has not yet been recommended in international guidelines. Therefore, every patient must be thoroughly counseled concerning the risks and benefits of this procedure (2).
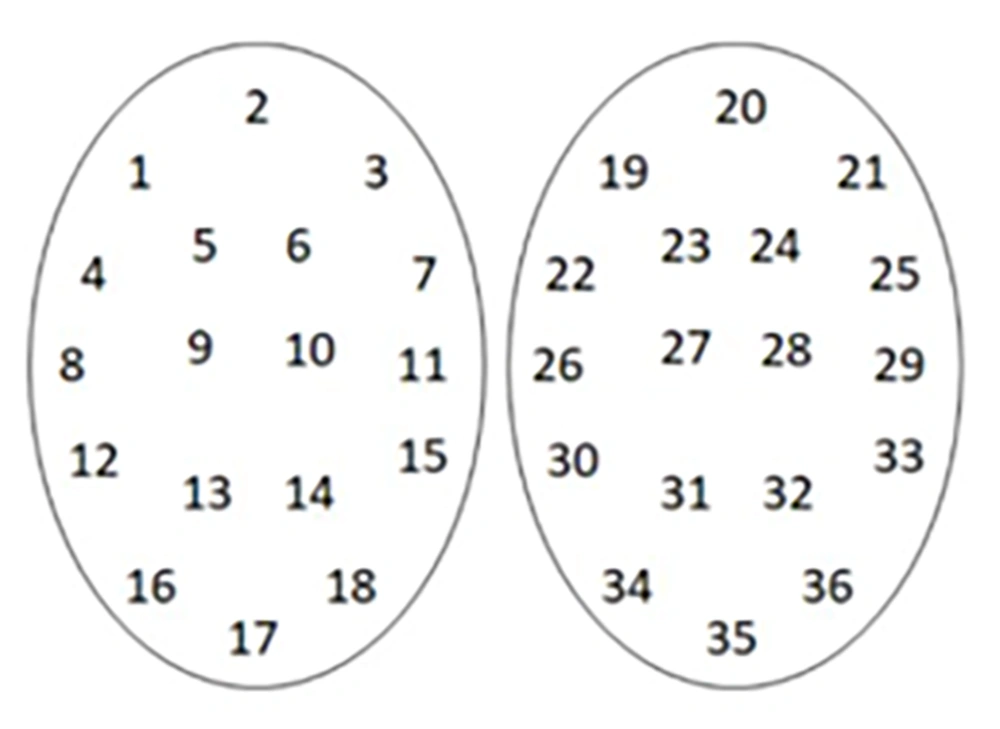
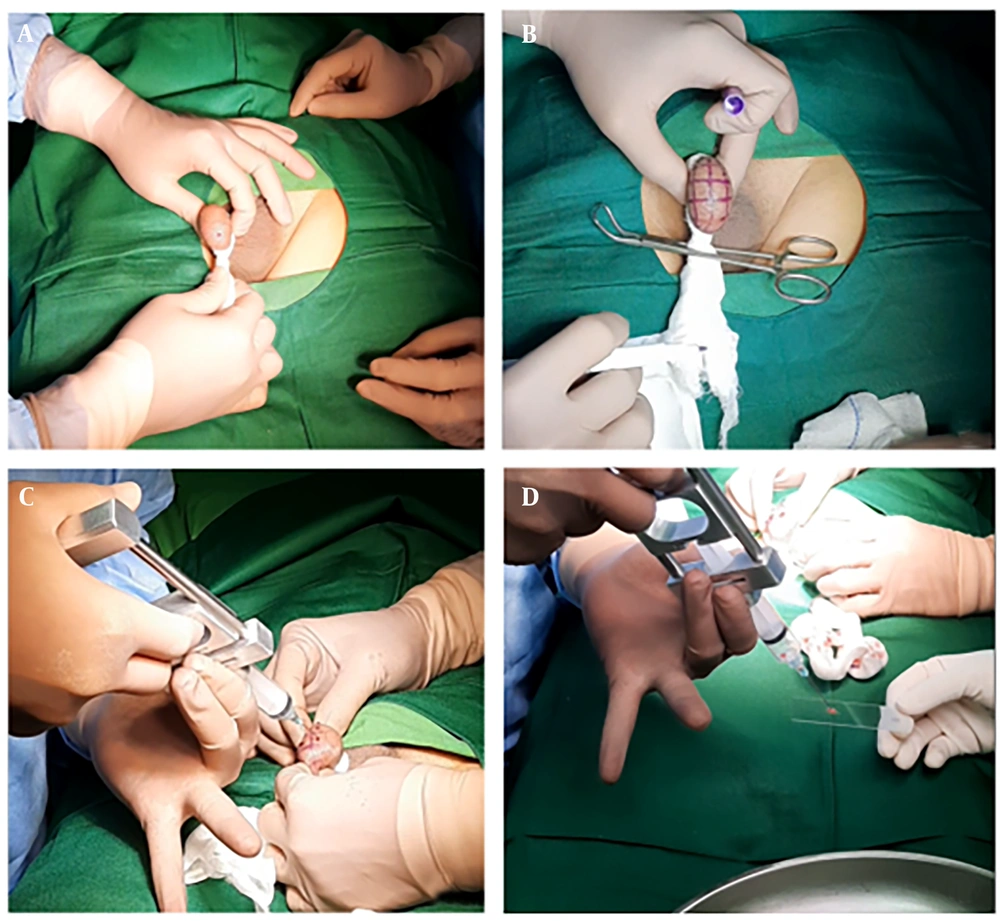
Testicular mapping biopsy is performed by systematically sampling 36 different testis sites (18 in each testis) (Figure 1). Thorough preparation of the surgical tools and materials is essential for the procedure (Figure 2). The procedure is performed by using a cutting and sucking motion with a 23G fine needle (Figure 3) (16). The samples from each site are examined to identify the presence of spermatozoa. The information provided by this procedure is valuable for identifying focal spermatogenesis in the testis of NOA patients, thus enabling targeted sperm retrieval techniques after the procedure. If spermatozoa are found in one site, microTESE has an 81% success rate. If there are two or more sites, TESE has a 90% success rate, and in all sites, TESA reaches 96% (2).
From the testicular biopsy, we can also assess the ability of seminiferous tubules with the presence of the maturity level of spermatozoa found in the biopsy tissue, which is established as the Johnsen score (Table 2) (17).
| Johnsen Score | Description |
|---|---|
| 10 | Complete spermatogenesis with many spermatozoa |
| 9 | Many spermatozoa present but germinal Epithelium disorganized with marked sloughing or obliteration of lumen |
| 8 | Only a few spermatozoa (< 5 - 10) present in section |
| 7 | No spermatozoa but many spermatids present |
| 6 | Only a few spermatids present (< 5 - 10) present |
| 5 | No spermatozoa, no spermatids but several or many spermatocytes present |
| 4 | Only few spermatocytes (< 5) and no spermatids or spermatozoa present |
| 3 | Spermatogonia are the only germ cells present |
| 2 | No germ cells but sertoli cells are present |
| 1 | No cells in tubular section |
1.5. Sperm Retrieval Success
Shefi et al. (18) studied sperm retrieval success rates in various pathologies causing NOA. The success rate reached 100% in patients with epididymo-orchitis and mumps-orchitis, while conditions like varicocele, cryptorchidism, and testicular torsion had success rates just below 70%. By utilizing testicular mapping biopsy, Jarvis et al. (16) created a heat map indicating the probability of sperm retrieval in patients who had previously undergone TESE/microTESE. This study found that focal spermatogenesis was most commonly located in the upper-lateral region of the testis, specifically at sites 4, 8, 12, and 13 on the right testis, and sites 20, 12, 24, and 25 on the left testis. Furthermore, a 100% sperm retrieval rate was observed in patients who underwent testicular mapping biopsy after failed TESE/microTESE. In contrast, conventional TESE or microTESE is typically performed on the equator of the testis, which may explain the failure rates of these methods. Testicular mapping biopsy allows for more tailored, less invasive sperm retrieval procedures with higher success rates (13).
1.6. Mapping Biopsy Risks
Testicular mapping biopsy is not without risks. The potential complications of any multiple biopsy procedures include infection, hematomas, testicular atrophy caused by devascularization of the testis, and hypogonadism due to testicular injury. However, the overall safety profile of this technique is well tolerated (19, 20). A study shows that three out of 85 azoospermic men reported prolonged pain that lasted 3 days after testicular mapping biopsy (21). Testicular ultrasound was then performed and revealed normal testicular ultrasound (20). Westlander et al. reported that 6% of patients developed focal intratesticular lesions 6 - 9 months after sperm aspiration with a large needle, without changes in testicular volume, follicle-stimulating hormone (FSH), or testosterone (22). Similar risk assessments are needed post-mapping biopsy. Jarvis et al. noted two mapping biopsy complications: Spermatic cord hematoma (1.2%) and painless hematospermia (3.65%), both resolving within a week (16).
1.7. Research Gap in the Indonesian Context
1.7.1. Mapping Biopsy Experience in Indonesia
Testicular mapping biopsy has been developed since 1997 in the USA. However, the experience using this procedure in Indonesia is still lacking. The first targeted TESE using microTESE following testicular mapping biopsy in a NOA patient in Indonesia was reported by Birowo et al. in 2020 (23). In the same year, we performed our first successful testicular mapping biopsy in a NOA patient.
1.8. Objective of the Present Case Series
The objective of this case series was to evaluate the feasibility and effectiveness of testicular mapping biopsy in NOA patients in Indonesia. This study aims to report the outcomes of sperm retrieval using this technique and assess its potential in providing tailored sperm retrieval options for patients with focal spermatogenesis. Furthermore, the case series seeks to highlight the clinical implications of using testicular mapping biopsy as an alternative to traditional sperm retrieval techniques, particularly for those with previously failed TESE or microTESE. By reporting our experience in Indonesia, this study intends to contribute to the growing body of evidence supporting the use of testicular mapping biopsy in NOA treatment and to serve as a foundation for future prospective studies in the region.
2. Case Presentation
2.1. Case 1 (IWPAP)
A 25-year-old patient came to our hospital with primary infertility after 1.5 years of marriage. He denied any erection or ejaculation problems. His wife was 25 years old without any gynecological problems. The couple had regular sexual intercourse around 3 times per week with no lubricant. The patient had a history of varicocele but hadn’t undergone any varicocele repair procedures. Physical examination showed normal testicles with bilateral varicocele. This result was confirmed by testicular ultrasound.
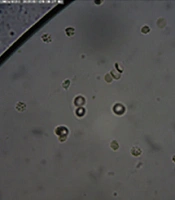
Laboratory results showed that the patient had azoospermia. Sperm analysis was done repeatedly and showed the same results. The patient had a normal FSH level and a negative Y-chromosome microdeletion (YCM). The patient elected to undergo sperm retrieval with PESA and TESE. Spermatozoa were found in several microscopic high-power fields. Hence, the samples were not able to be cryopreserved for future In Vitro Fertilization. The testicular biopsy showed that the Johnson Score was 7.0 for both testicles. The patient consented to a testicular mapping biopsy, during which non-motile spermatozoa were found in sites 3, 23, 26, 2, and 36 (Figure 4), with no adverse effects. Then, the patient was counseled for the possibility of hormone therapy prior to a targeted sperm extraction procedure 3 months after the testicular mapping biopsy to optimize results in the future. However, after 1 and a half years, the patient hadn’t returned to our clinic.
2.2. Case 2 (PEH)
A 33-year-old patient came to our hospital with primary infertility after 5 years of marriage. He reported no erection or ejaculation problems. His 31-year-old wife had no gynecological issues, and they had regular weekly intercourse without lubricant. The patient had a history of orchitis in his adolescence and varicocele two years prior to coming to our clinic. He had undergone a testicular biopsy at that time and had the varicocele repaired one year later. Physical examination and ultrasound showed normal testes without varicocele.
Laboratory results showed that the patient had azoospermia. The testicular biopsy result indicated "Sertoli cells only syndrome" with a Johnson score of 2.0 - 2.0. The patient had a normal FSH level and a negative YCM but a positive gr/gr deletion in his AZFc region. He elected to undergo a testicular mapping biopsy. No spermatozoa were found in the 36 sites of biopsy mapping (Figure 5). There were no adverse effects observed afterward. With that result, further plans for assisted reproductive methods couldn’t be proceeded.
2.3. Case 3 (LTH)
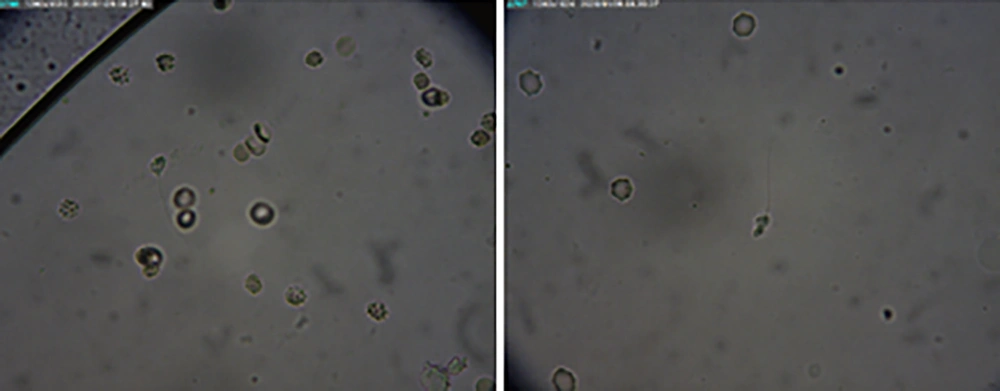
A 38-year-old patient came to our hospital with primary infertility after 11 years of marriage. He had no issues with erection or ejaculation. His 38-year-old wife had no gynecological problems. The couple had regular sexual intercourse 2 - 3 times per week with no lubricant. He had childhood orchitis and underwent varicocelectomy in 2016 in Samarinda, Indonesia. Physical examination showed normal testes and bilateral grade II varicocele. This was confirmed by testicular ultrasound. Laboratory results showed that the patient had azoospermia. Sperm analysis was repeated, and the second sperm analysis also showed azoospermia. The patient had normal hormonal parameters and a negative YCM. The patient elected to undergo sperm retrieval with PESA, but no motile spermatozoa were retrieved. The TESE was then performed, and 1 - 2 non-motile spermatozoa were found in several high-power fields. Hence, the sperm sample was not able to be cryopreserved for future In Vitro Fertilization. A testicular biopsy sample was obtained during TESE and was sent to the histopathology lab for further analysis. Biopsy during TESE showed Johnsen scores of 8 (right) and 7 (left). The patient later underwent a testicular mapping biopsy, which found non-motile spermatozoa at sites 6, 10, 11, 23, 31, 35, and 36 without adverse effects observed afterward (Figure 6). He was counseled on potential hormone therapy prior to targeted sperm extraction in 3 months to improve outcomes.
2.4. Case 4 (AS)
A 56-year-old patient came to our hospital with secondary infertility after 1 month of marriage. He had a 25-year-old child from his first marriage. This is his second marriage. He denied any erection problems; however, the patient occasionally suffers from painful ejaculation. His 25-year-old wife had no gynecological problems. The couple engaged in regular intercourse twice weekly without lubricant. The patient had well-controlled hypertension and no other known medical history.
Laboratory results showed that the patient had azoospermia. Sperm analysis was repeated, and the second sperm analysis also showed azoospermia. The patient had abnormal hormonal parameters (FSH 26.31; LH 8.89; testosterone 4.36; prolactin 9.6) and a negative YCM. Scrotal ultrasound showed homogeneous normal testes (right testicular volume 8 cc; left testicular volume 7 cc), dilated epididymis in the right testis (1.32 × 1.41 cm), and bilateral varicocele.
The patient elected to undergo sperm retrieval with PESA. However, no motile spermatozoa were found during the procedure. The TESE was then performed, but unfortunately, no spermatozoa were found during the procedure. A testicular biopsy taken during TESE revealed a Johnsen score of 5 in both testicles. He was then counseled for a testicular mapping biopsy. The patient elected to undergo a testicular mapping biopsy. Non-motile spermatozoa were found at site 12 during the procedure (Figure 7). There were no adverse effects observed afterward.
2.5. Case 5 (ANA)
A 31-year-old man presented with primary infertility after 1 year of marriage. He reported no erectile or ejaculatory issues. His 28-year-old wife had no gynecological problems. The couple had regular sexual intercourse 2 - 3 times per week with no lubricant. The patient had no history of urological problems before. Physical examination and ultrasound showed normal testicles with mild (grade I) left varicocele.
Laboratory results showed that the patient had azoospermia. Sperm analysis was repeated several times and showed the same results. The patient had normal hormonal parameters and a negative YCM. The patient elected to undergo sperm retrieval. The PESA and TESE were then performed, but no spermatozoa were found in several microscopic high-power fields. Hence, the samples were not able to be cryopreserved for future In Vitro Fertilization. Biopsy showed a Johnsen score of 2 in both testes. He was then counseled for a testicular mapping biopsy. The patient then underwent a testicular mapping biopsy, which showed no sperm in all 36 sites. No adverse effects were observed afterward. Due to these findings, further assisted reproductive options could not be pursued.
2.6. Case 6 (HHT)
A 37-year-old patient came to our hospital with primary infertility after two years of marriage. He had no erection or ejaculation issues. His 32-year-old wife had no gynecological problems. The couple had regular intercourse 1 - 2 times per week without lubricant. He had a history of kidney stones treated with ESWL in 2016. Physical examination and ultrasound showed bilateral testicular hypotrophy without varicocele.
Laboratory results showed that the patient had azoospermia. Sperm analysis was repeated several times and showed the same results. The patient had a high level of FSH. No YCM was found. The patient had consulted about IVF in Singapore before he came to our hospital. He was recommended to have microTESE. After further consultation, he underwent a testicular mapping biopsy, which revealed a single non-motile spermatozoon at site 25 (Figure 8). There were no adverse effects observed afterward. He was advised to consider hormonal therapy and antioxidant supplementation prior to a targeted sperm extraction procedure 3 months after the testicular mapping biopsy to optimize results before planning to undergo a microTESE procedure in the future.
2.7. Case 7 (WD)
A 41-year-old patient came to our hospital with secondary infertility after 16 years of marriage. They have an eleven-year-old child. He denied any erection or ejaculation problems. His 43-year-old wife had no gynecological issues. He had no prior ejaculation problems but developed erectile dysfunction following a motorcycle accident 3 years ago that caused paralysis below the lumbar 2 region. Physical examination and ultrasound showed normal testes with bilateral varicocele.
Laboratory results showed that the patient had azoospermia. Sperm analysis was repeated several times and showed the same results. The YCM analysis was not performed, as the patient had a child previously. The patient elected to undergo sperm retrieval with PESA and TESE, but no spermatozoa were found. Hence, the samples were not able to be cryopreserved for future In Vitro Fertilization. Testicular biopsy showed a Johnsen score of 4 in both testes. Later, the patient elected to undergo bilateral varicocele microligation.
He was then counseled for a testicular mapping biopsy. The patient agreed to undergo a testicular mapping biopsy. No sperm were found in any of the 36 sites of testicular biopsy mapping. There were no adverse effects observed afterward. Hence, no further plan for assisted reproductive methods could be performed.
2.8. Ethical Considerations
This study was based on secondary data obtained from patient medical records and procedural documentation. All patients included in the study provided written informed consent for their anonymized clinical data to be used for research and publication purposes. The study was reviewed and approved by the Institutional Review Board (IRB) of the Faculty of Medicine, Universitas Indonesia – Cipto Mangunkusumo Hospital (approval No: KET-1234/UN2.F1/ETIK/PPM.00.02/2023).
3. Discussion
The NOA is often considered the most severe spectrum of male infertility. The etiology of azoospermia is idiopathic in 13.3% of cases; hence, complete screening to determine the underlying cause for NOA is compulsory. Ideally, all underlying causes should be treated before the sperm retrieval procedure (1).
Sperm retrieval in NOA is a very complex matter. Focal spermatogenesis in NOA has made it very difficult for surgeons to achieve sperm retrieval success. The best sperm retrieval method available is microTESE, with a sperm retrieval rate of 42% - 63%. As good as it may be, the odds are still not satisfying. Testicular mapping biopsy has provided a solution to this matter by taking a minimal amount of testicular tissue to create a map of the patient's testis. This "map" will then be used for future sperm retrieval procedures, enabling a less invasive, targeted, and tailored procedure for each patient (2, 13). Moreover, testicular mapping biopsy has been proven to successfully extract sperm from patients with previously failed TESE/microTESE (16).
Our first experience with Testicular Mapping Biopsy in Indonesia shows promising results, with a 57.14% (4/7) sperm retrieval success rate. Targeted sperm retrieval can be performed in these patients after thorough counseling. Among all cases, four had undergone previous TESE, and two out of these four (50%) patients had successful sperm retrieval by testicular mapping biopsy after previously failed TESE. Our results are coherent with previous findings by Shefi et al., who reported an overall sperm retrieval rate for testicular mapping biopsy of 67.85% (18). This study further analyzed the sperm retrieval rate for known testicular pathologies, with the highest rates for epididymo-orchitis and mumps orchitis (100%), followed by cryptorchidism (69%), testicular torsion (67%), and the least successful being varicocele (63%) (18).
Based on recommendations from the EAU Guideline, further andrological assessment is recommended when there are at least two abnormal sperm analyses (1). We performed these assessments and found different underlying causes for each of our cases. Of all patients, only case 4 presented with secondary infertility. Every case's sperm analysis showed azoospermia. Among all patients, three out of six cases (50%) presented with abnormal hormonal parameters, specifically cases 2, 4, and 6. Varicocele was present in all cases except case 6. Case 5 had left-sided varicocele only, and case 3 had bilateral recurrent varicoceles. The rest of the cases had bilateral varicocele. In a study in Europe, varicocele is responsible for 14.8% of all male infertility cases and 10.9% of all azoospermic patients. A meta-analysis by Birowo et al. summarized the benefits of varicocele repair for male infertility, showing a significant increase in pregnancy rate with an OR of 1.82 (95% CI 1.37 - 2.41) (24). Among the azoospermia subgroup, the benefit favors varicocele repair with an OR of 2.34 (95% CI 1.03 - 5.35), although these findings are not statistically significant. Among patients with subnormal semen parameters, varicocele repair increases the pregnancy rate with an OR of 1.74 (1.27 - 2.38) (24).
The detailed clinical, hormonal, genetic, and histopathological characteristics of the seven cases are summarized in Table 3. In terms of varicocele, its presence may impair spermatogenesis through mechanisms such as elevated scrotal temperature, hypoxia, and oxidative stress. Some patients in our series had clinical varicocele but did not undergo varicocelectomy prior to mapping, which might have influenced their sperm retrieval outcomes. Although varicocelectomy has been shown to improve spermatogenesis in selected NOA patients, the timing of mapping — whether before or after varicocelectomy — remains controversial. We acknowledge this variation as a limitation and have added a paragraph in the Discussion to emphasize the need for standardized pre-mapping assessment and treatment of varicocele. Further studies are warranted to investigate the impact of prior varicocelectomy on mapping biopsy outcomes.
| Case | Patient Initials | Infertility | Sperm Analysis | FSH | LH | Testosterone | Varicocele | Testicular Biopsy | YCM | gr/gr Deletion | Testicular Mapping Biopsy Result | Previous TESE Result | Adverse Effect |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IWPAP | Primary | Azoospermia | 2.45 | 6.10 | 4.07 | Bilateral varicocele | 7.0, 7.0 | Negative | No deletion | Non-motile spermatozoa were found in site 3, 23, 26, 28 36 | No spermatozoa was found | No adverse effect |
| 2 | PEH | Primary | Azoospermia | 15.78 | 3.95 | 3.39 | Bilateral varicocele | - | Negative | +/+ | No spermatozoa was found | - | No adverse effect |
| 3 | LTH | Primary | Azoospermia | 3.9 | 3.7 | 4.59 | Bilateral recurrent varicocele | 8.0, 7.0 | Negative | Not yet | Non-motile spermatozoa were found in sites 6,10,11,23 ,31,35,36 | 1 - 2 non motile spermatozoa were found in several high-power field | No adverse effect |
| 4 | AS | Secondary | Azoospermia | 26.31 | 8.89 | 4.36 | Bilateral varicocele | 5.0, 5.0 | Not yet | Not yet | Non-motile spermatozoa was found in site 12 | No spermatozoa was found | No adverse effect |
| 5 | ANA | Primary | Azoospermia | 4.99 | 2.63 | 7.41 | Left varicocele | 2.0, 2.0 | Negative | No deletion | Not Found | No spermatozoa was found | No adverse effect |
| 6 | HHT | Primary | Azoospermia | 16.06 | 6.01 | 5.00 | No | - | Negative | Not yet | Non-motile spermatozoa was found in site 25 | - | No adverse effect |
| 7 | WD | Secondary | Azoospermia | 20.8 | 48.7 | 4.9 | Bilateral varicocele | 4.0, 4.0 | Negative | Not yet | Not found | No spermatozoa was found | No adverse effect |
Abbreviations: FSH, follicle-stimulating hormone; LH, luteinizing hormone; YCM, Y-chromosome microdeletion; TESE, testicular sperm extraction.
Testicular biopsy was performed in five cases, as recommended by the EAU guideline (1). The testicular biopsy results were classified using the Johnsen score (Table 2). Among our cases, the most severe testicular histopathology result was a Johnsen score of 2.0 for both the right and left testes, indicating no germ cells present. The YCM analysis was performed in five cases, all of which presented with negative YCM. Y-chromosome deletion is a known etiology for male infertility, and these deletions were not found among men with normal sperm analysis. The YCM is very rare in men with sperm concentrations of > 5 million/mL but is commonly found in men with azoospermia (8 - 12%) and men with oligospermia (3-7%) (1). We performed YCM analysis as recommended by the EAA/EMQN best practice guideline (25).
Regarding the discrepancy between higher Johnsen scores and failed sperm retrieval: Although the Johnsen score reflects the degree of spermatogenic activity in examined seminiferous tubules, it does not fully represent the entire testicular architecture, particularly in patients with NOA. Spermatogenesis in NOA is known to be patchy and focal, and testicular mapping may miss isolated foci of sperm-producing regions despite relatively high histological scores. Additionally, mechanical limitations during aspiration, tissue fragmentation, or lab handling may influence the final outcome of sperm detection.
We performed gr/gr deletion screening in three cases, with one case showing a positive gr/gr deletion (case 2). Gr/gr deletion is a new type of Yq deletion described in the AZFc region (1). This particular region is very susceptible to non-allelic homologous recombination (NAHR), resulting in the formation of partial deletions/duplications. Previous studies have shown that deletion in this specific site increases the risk for oligozoospermia by 2.5 - 8 times and also increases the risk for infertility (25). Moreover, a meta-analysis study from Italy has shown gr/gr deletion as a significant risk factor for poor sperm production with an OR of 7.9 (95% CI 1.8 - 33.8) (26). There is still a debate among experts about whether routine screening for gr/gr deletion should be performed for infertile males (1).
No adverse effects were reported in all cases. While previous studies noted complications such as hematomas, infection, hypogonadism, and testicular devascularization, the only notable adverse effect was prolonged pain for three days in 3 out of 85 patients who had undergone testicular mapping biopsy (19, 20). A retrospective study reported two different complications after testicular mapping biopsy: Spermatic cord hematoma (1.2%) and painless gross hematospermia (3.65%). None of these complications occurred in our cases.
From our experience with testicular mapping biopsies, we observed several advantages and disadvantages during the procedures, which are summarized in Table 4.
| Advantages | Disadvantages |
|---|---|
| Higher accuracy for sperm retrieval | The necessity of fresh egg retrieval, female partner may undergo unnecessary procedures |
| Less postoperative complications (hematoms, infections) | Time-consuming for next sperm retrieval |
| Minimum testicular atrophy and hypogonadism risk | Costly compared to only conventional sperm retrieval |
This study is a case series with a limited sample size, as testicular mapping biopsy has only been performed seven times in Indonesia. Additionally, due to the retrospective design and real-world clinical constraints, diagnostic workups were not standardized across all cases. For example, YCM testing was not conducted for cases 4 and 7, and the gr/gr deletion test was performed in only three patients. The grading of varicocele was inconsistent, and some data were missing. Specifically, data for case 2 was not available and has been clearly indicated in the table with a footnote stating "Data not available". Furthermore, follow-up data regarding the use of retrieved sperm in assisted reproductive techniques such as IVF or ICSI were unavailable, mainly due to delays in patient coordination with fertility clinics and individual preferences. These limitations highlight the need for larger, prospective studies with standardized diagnostic evaluations and long-term follow-up to assess the full clinical impact of testicular mapping biopsy in NOA treatment. This limitation has also been acknowledged in the Discussion section to ensure transparency.
3.1. Conclusions
Particularly in the Indonesian context, testicular mapping biopsy presents a viable substitute for sperm retrieval in cases of NOA. This study indicates that testicular mapping biopsy has a sperm retrieval rate similar to TESE and microTESE, despite being observational, descriptive, and having a small sample size. For patients who have had unsuccessful TESE or microTESE attempts at sperm retrieval in the past, the procedure offers a feasible alternative. While the procedure is generally well tolerated, more research is required to fully evaluate the risks and long-term effects, including any effects on testosterone levels and hypogonadism, which were not systematically evaluated in this study. Future studies should examine the wider use of testicular mapping biopsy in a variety of settings and monitor testosterone levels after the procedure.