1. Background
Hypernatremia, a common clinical finding, arises from various etiologies, including pure water loss (e.g., diabetes insipidus), hypotonic fluid loss (e.g., gastrointestinal losses), hypertonic sodium gain (e.g., iatrogenic causes), and inadequate intake (e.g., lactation insufficiency) (1). In a normal physiological state, a dynamic equilibrium exists between the extracellular fluid (ECF) and intracellular fluid (ICF) compartments. Hypernatremia disrupts this equilibrium by increasing ECF tonicity, primarily due to elevated sodium concentration (2). The hypertonic ECF state creates an osmotic gradient. This gradient draws water from the ICF into the ECF, resulting in cellular dehydration and shrinkage. This continues until osmotic equilibrium is re-established across the cell membrane (3). Chronic hypernatremia, lasting days to weeks, elicits cellular adaptations to mitigate dehydration. Cells accumulate intracellular osmolytes — small organic solutes — to maintain osmotic balance between intracellular and ECF. However, cerebral tissue adaptation is slower, taking approximately a week to near-fully preserve intracellular water content. This slower cerebral adaptation minimizes water loss from brain cells, preserving cell volume and stabilizing intracellular proteins. This difference in adaptation rates between brain and peripheral tissues has crucial clinical implications during hypernatremia correction. Rapid correction in peripheral tissues restores cell volume effectively. Conversely, rapid serum sodium reduction in the brain can cause cerebral edema due to persistent intracellular osmolytes creating an osmotic gradient that draws water into the brain, resulting in swelling (4, 5). While the body's adaptive mechanisms help mitigate cellular dehydration, the unique physiology of neonates poses distinct challenges in managing hypernatremia. Given their immature renal function and limited ability to regulate sodium balance effectively, neonates are particularly vulnerable to prolonged hypertonicity. Furthermore, the clinical management of neonatal hypernatremia is complicated by the need for careful sodium correction to prevent complications such as cerebral edema. The clinical approach to neonatal hypernatremia involves rehydration, where the replacement volume is calculated using the water-deficit equation combined with maintenance fluid requirements. Traditionally, 1.5 times the maintenance fluid is administered over the first 24 hours, with close monitoring of water and electrolyte levels (6). The previous preference for hypotonic fluids stemmed from a concern that infants' kidneys might have limited ability to excrete sodium efficiently, potentially leading to hypernatremia if isotonic fluids were given. However, newer guidelines recommend isotonic fluids due to their closer resemblance to plasma, while also emphasizing a more individualized approach based on clinical condition (7).
2. Objectives
Given the variability in neonatal hypernatremia management across hospital settings due to the complexity of the management, this study investigated a practical, simplified treatment approach for hypernatremic dehydration.
3. Methods
3.1. Study Design and Population
This prospective observational cohort study enrolled neonates presenting to the pediatric emergency department at Children's Medical Center between October 2022 and October 2024. Neonates with clinical dehydration due to decreased oral intake underwent screening for hypernatremia. Serum sodium levels were measured using an ion-selective electrode (ISE) method in the hospital central laboratory. Hypernatremia was defined as a serum sodium concentration > 150 mEq/L on initial presentation (8). A standardized intervention protocol was implemented for all neonates presenting to the emergency department with hypernatremia. Following resuscitation, these neonates were transferred to the neonatal intensive care unit (NICU).
3.1.1. Inclusion Criteria
This study included all neonates younger than 28 days of age with a gestational age of ≥ 37 weeks who presented with dehydration and a serum sodium concentration > 150 mEq/L.
3.1.2. Exclusion Criteria
Neonates were excluded if their hypernatremia developed within a few hours of presentation as acute hypernatremia, was attributed to iatrogenic causes, or was secondary to diarrhea or diabetes insipidus.
3.2. Rehydration Protocols
3.2.1. Resuscitation Treatment
Oliguric neonates received a 10 mL/kg bolus of normal saline intravenously over 30 minutes. Urinary output was assessed after 30 minutes. A second and third identical bolus was administered, followed by another 30-minute observation period if urination did not occur. Concurrent blood samples were collected for electrolyte analysis and other laboratory tests.
3.2.2. Maintenance Treatment
Following confirmation of diuresis, a post-resuscitation fluid replacement regimen was initiated. This regimen used 1.2 to 1.5 times the standard maintenance fluid requirement, adjusted for dehydration severity. The standard maintenance fluid requirement was defined as 120 mL/kg/day of 10% dextrose solution, with added maintenance sodium and potassium. The total volume of previously administered fluid boluses was subtracted from this calculated daily requirement to determine the adjusted replacement volume.
Neonatal patients with hypernatremia were stratified into three subgroups based on their initial serum sodium concentration. Subgroup 1: Serum sodium concentration 150 - 165 mEq/L, subgroup 2: 166 - 175 mEq/L, and subgroup 3: Serum sodium concentration ≥ 176 mEq/L.
Resuscitation fluid sodium concentration was tailored to each subgroup: 75 mEq/L for subgroup 1, 154 mEq/L for subgroup 2, and 10 mEq/L below the patient's measured serum sodium level for subgroup 3 (9). The 24-hour serum sodium replacement regimen was individualized, based on the patient's measured serum sodium concentration, assessed every 4 to 6 hours. The volume of initial resuscitation fluids was factored into the calculation of overall fluid balance. Breast milk was initiated when the serum sodium concentration fell below 150.
3.3. Outcomes
The primary outcome measure was the rate of plasma sodium reduction within 24 hours, defined as a maximum decrease of 10 mEq/L.
3.3.1. Secondary Outcomes
Based on the observed correlation between initial serum sodium concentration and the rate of correction, we hypothesized the following timeframes for normalization: (1) 24 hours for serum sodium levels of 150 - 165 mEq/L; (2) 48 hours for serum sodium levels of 166 - 175 mEq/L; (3) 72 - 6 hours for serum sodium levels of ≥ 176 mEq/L.
3.3.2. Tertiary Outcome
The tertiary endpoint assessed the prevention of fluid therapy-related adverse events, including fluid overload (manifested as weight gain, hypertension, or edema) and neurological complications from rapid sodium correction (such as brain edema or seizures).
3.4. Ethics
This study was performed in line with the principles of the Declaration of Helsinki. Informed consent for participation and publication was obtained from the parents of the infants. In addition, the Ethics Committee of Tehran University of Medical Sciences approved this study (ethics code: IR.TUMS.CHMC.REC.1401.078).
3.5. Statistics
Baseline demographic and clinical characteristics were described using descriptive statistics. Laboratory data obtained throughout hospitalization were subjected to comparative analysis across the three subgroups. Analysis of variance (ANOVA) and post-hoc tests were employed to evaluate differences in sodium drops among the subgroups. A significance threshold of P < 0.05 was adopted for all statistical tests. Statistical analyses were performed using IBM SPSS Statistics version 24.
4. Results
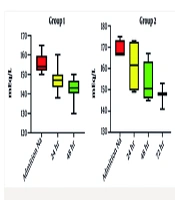
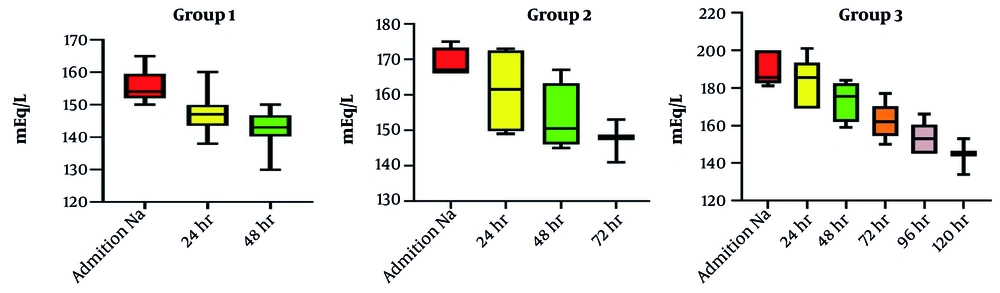
This study investigated 43 neonates stratified into three groups based on initial serum sodium concentration: Group 1 (n = 33), group 2 (n = 4), and group 3 (n = 6). Table 1 presents descriptive statistics (demographics and characteristics) for each group. Table 2 shows the clinical presentations of the neonates. Table 3 presents the initial serum sodium concentrations on emergency room presentation, time to serum sodium < 150 mEq/L, and the magnitude of sodium reduction during fluid correction. Analysis of variance showed no statistically significant difference in the rate of sodium decline among groups (daily reduction consistently < 10 mEq/L in all groups). Time to achieve serum sodium < 150 mEq/L varied: Group 1 (≤ 48 hours), group 2 (≤ 72 hours), and group 3 (≤ 6 days). Two patients died: One in group 1 with an initial presentation of ichthyosis, immunodeficiency, recurrent infection, and deep vein thrombosis, and one in group 3 (serum sodium 180 mEq/L) with an initial presentation of cavernous sinus thrombosis, acute tubular necrosis, and seizures. Rehydration therapy was generally effective; the only complication was a seizure in one of the group 3 patients, who was discharged, and after 4 months of follow-up, he had normal development and was discontinued from anti-epileptic drug therapy.
| Variables (N = 43) | Group 1 (n = 33) | Group 2 (n = 4) | Group 3 (n = 6) |
|---|---|---|---|
| Sex (male); no. (%) | 20 (60.6) | 1 (25) | 4 (66.6) |
| Delivery route (C/S); no. (%) | 21 (63.6) | 4 (100) | 4 (66.6) |
| Birth weight; g (IQR) | 3170 (2957.5, 3377.5) | 3072.5 (2693.7, 3417.5) | 3200 (2275, 3900) |
| Gestational age; wk (IQR) | 38 (37, 39) | 38.5 (37.25, 37.75) | 38 (37.5, 38.5) |
| Admission weight; g (IQR) | 2900 (2700, 3300) | 2750 (2547.5, 3275) | 2215 (2050, 2900) |
| Discharge weight; g (IQR) | 3055 (2720, 3298.75) | 2757.5 (2550, 3163.75) | 3000 (1087.5, 3187.5) |
| Age of admission; d (IQR) | 5 (3.5, 15) | 14 (8, 71) | 26 (12.24, 40.5) |
Demographic and Characteristics of Neonates
| Variables (N = 43) | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| Oliguria | 5 | - | 2 |
| Fever | 16 | 4 | - |
| Icterus | 10 | - | 1 |
| Lethargy | 2 | 1 | 2 |
| Poor feeding | 7 | 1 | 3 |
| Seizure | 2 | - | - |
| Thrombosis | 1 | - | 1 |
Initial Presentation of Hypernatremia Dehydration
| Variables (N = 43) | Group 1 (n = 33) | Group 2 (n = 4) | Group 3 (n = 6) | P-Value |
|---|---|---|---|---|
| Na admission | 154 (152, 159.5) | 167 (166, 173.25) | 185.5 (182.5, 200) | - |
| Na drop; 24 h | 9 (5.5, 13) | 9 (2.75, 16.9) | 4.5 (1.75, 9.75) | N/S c |
| Na serum; 48 h | 150 (148, 154) | 161 (149, 172.5) | 185.5 (169, 193) | - |
| Na drop; 48 h | 8 (5, 12) | 5 (3.25, 15.75) | 7 (6, 14) | N/S c |
| Na serum; 72 h | 148 (146, 148) | 152 (150, 159) | 175 (162, 182.5) | - |
| Na drop; 72 h | 3.5 (1.5, 9.25) | 8 (6, 11) | 9 (6, 15.5) | N/S c |
| Na serum; 96 h | - | - | 165 (157.5, 172) | - |
| Na drop; 96 h | - | - | 11 (7.5, 17.5) | N/S c |
| Na serum; 120 h | - | - | 155 (154, 160.5) | - |
| Na drop; 120 h | - | - | 19 (10, 20) | N/S c |
| Na serum; 144 h | - | - | 149 (143, 153) | - |
| Na drop; 144 h | - | - | 4 (1, 8) | N/S c |
5. Discussion
Management of neonatal hypernatremia requires a cautious and individualized approach, prioritizing slow correction to prevent cerebral edema. The process involves assessing severity, identifying the cause, determining the serum sodium level, and identifying the underlying cause (e.g., inadequate fluid intake, diarrhea, diabetes insipidus, and renal losses). This dictates urgency and treatment specifics (10). For hypernatremic dehydration treatment, it is necessary to calculate water deficit using the water-deficit equation: Water deficit (liters) = Total body water (TBW) × [(Na+ - 140)/140], where Na+ is serum sodium. However, estimating TBW in neonates requires careful consideration of gestational age, postnatal age, and weight. Various formulas exist, and clinical judgment is essential. The sodium level should never be lowered by more than 0.5 to 1 mEq/L per hour to prevent cerebral edema. This slow correction allows gradual equilibration of intracellular and ECF. Total correction might take 24 - 48 hours or longer, depending on severity. Rapid correction is dangerous (11, 12).
Hypernatremia in neonates presents significant clinical challenges, given the variability in neonatal hypernatremia management across hospital settings due to the complexity of the management; this study investigated a practical, simplified treatment approach for hypernatremic dehydration. Durrani et al. introduced a practical approach to the management of hypernatremia in neonates (2). Their method was very accurate and convenient, but it is difficult to perform, especially for beginner doctors, and there is a high probability of error. The findings of this study contribute valuable insights into the management of neonatal hypernatremia, particularly emphasizing the safety and efficacy of a simplified treatment approach.
The study's primary outcome demonstrated that the rate of plasma sodium reduction remained consistent across the three groups, with all groups achieving a daily decrease of less than 10 mEq/L. The absence of statistically significant differences in sodium decline among the groups suggests that our treatment approach, which emphasizes careful monitoring and gradual correction, is effective regardless of the initial sodium concentration.
Our secondary outcomes, which hypothesized timeframes for normalizing serum sodium levels based on initial concentrations, revealed that group 1 (sodium levels 150 - 165 mEq/L) typically achieved target levels within 48 hours, while groups 2 and 3 demonstrated longer timeframes corresponding to their higher initial sodium levels, as shown in Figure 1. Several factors contributed to prolonged sodium normalization in some patients. Patients with higher initial serum sodium concentrations (≥ 176 mEq/L) required more extended correction periods, sometimes up to six days. The more severe the hypernatremia, the longer the time needed for sodium levels to normalize safely. Individualized rehydration protocols aimed at gradual sodium reduction (< 10 mEq/L per day) were essential to prevent complications like cerebral edema. However, patients with extremely high sodium levels required lower sodium concentrations in their resuscitation fluids, which may have slowed normalization. This finding emphasizes the need for tailored management strategies based on the severity of hypernatremia, reinforcing the notion that a one-size-fits-all approach may not be appropriate in neonatal care (13).
The severity of hypernatremia predisposed some patients to serious complications. One neonate in group 3 (serum sodium 180 mEq/L) with an initial presentation of cavernous sinus thrombosis, acute tubular necrosis, and seizures ultimately led to mortality. Another patient experienced a seizure but recovered after treatment.
The tertiary outcomes of our study also warrant discussion. The overall safety profile of the treatment was encouraging, with only one seizure reported in a patient from the highest sodium group. This incident, although concerning, underscores the necessity for vigilant monitoring during treatment, particularly in cases with severe hypernatremia. The low incidence of complications such as fluid overload or rapid sodium correction-related neurological events supports the notion that our treatment protocol could be safely implemented in clinical practice (14).
The mortality rate observed in the study, with two deaths occurring in patients with significant underlying conditions, reflects the complexity of treating neonates with hypernatremia. Both cases had multiple comorbidities, indicating that while our treatment approach may effectively manage hypernatremia, the overall outcomes are also heavily influenced by the patients' baseline health status. This underscores the importance of a multidisciplinary approach in managing such critically ill neonates, where careful consideration of individual patient circumstances is paramount (15).
5.1. Conclusions
In conclusion, our study suggests that a practical and simplified treatment regimen for neonatal hypernatremia can be effective and safe, with favorable outcomes in most cases. The findings support the need for further research to validate our proposed timeframes for sodium normalization and to refine treatment protocols that accommodate the variability in clinical presentations.
5.2. Limitations
This study has several limitations, including a short follow-up period, single-center design, and a lack of control group, which restricts the generalizability of the findings. Future research involving larger multicenter cohorts and the long-term neurological outcomes of neonates treated for hypernatremia, as well as the impact of differing management strategies on overall morbidity and mortality, is needed.