1. Introduction
Membranous nephropathy (MN) is characterized by the formation of subepithelial immune deposit with resultant changes in glomerular basement membrane (GBM), most notably spike formation. Approximately 75% of MN represent as primary disease and the rest results from secondary causes, most commonly systemic lupus nephritis (SLE), infections such as hepatitis B or C viruses, malignancy, or drugs. Antineutrophil cytoplasmic antibodies (ANCA)-associated glomerulonephritis (GN) is characterized by necrotizing and crescentic GN with paucity of immunoglobulin (Ig) and complement deposition, which is also known as pauci-immune crescentic GN (1-3). We report a rare case of MN with myeloperoxidase (MPO)-ANCA-associated crescentic GN in a 48 year-old-man who was admitted to our institute.
2. Case Presentation
A 48-year-old man presented with intermittent puffiness of face and edema of the feet for two months. He had hypertension for two months, which was treated. He did not have fever, hematuria, or breathlessness. On examination, he had bilateral pitting pedal edema (+ +), pulse rate of 98 per minute, and blood pressure of 140/96 mm Hg. Cardiovascular and respiratory examinations were unremarkable. On investigation the following laboratory results were reported: hemoglobin, 6.1 gm/dL; white blood cell (WBC) count, 5.6 × 109/L; platelet count, 2.11 × 109/L; blood urea nitrogen, 35 mmol/L; serum creatinine, 807 μmol/L; random blood sugar, 5.33 mmol/L; total serum protein, 500 g/L; serum albumin, 31 g/L; serum sodium, 132 mmol/L; serum potassium, 4.54 mmol/L; and serum cholesterol, 5.28 mmol/L. Urine analysis showed 3 + albumin with 35 to 40/HPF of red blood cells and 8 to10/HPF of WBC. Results of viral screening for human immunodeficiency virus, hepatitis B and hepatitis C viruses were negative. Serum MPO-ANCA level was 220 U/mL (normal range, 1-5).
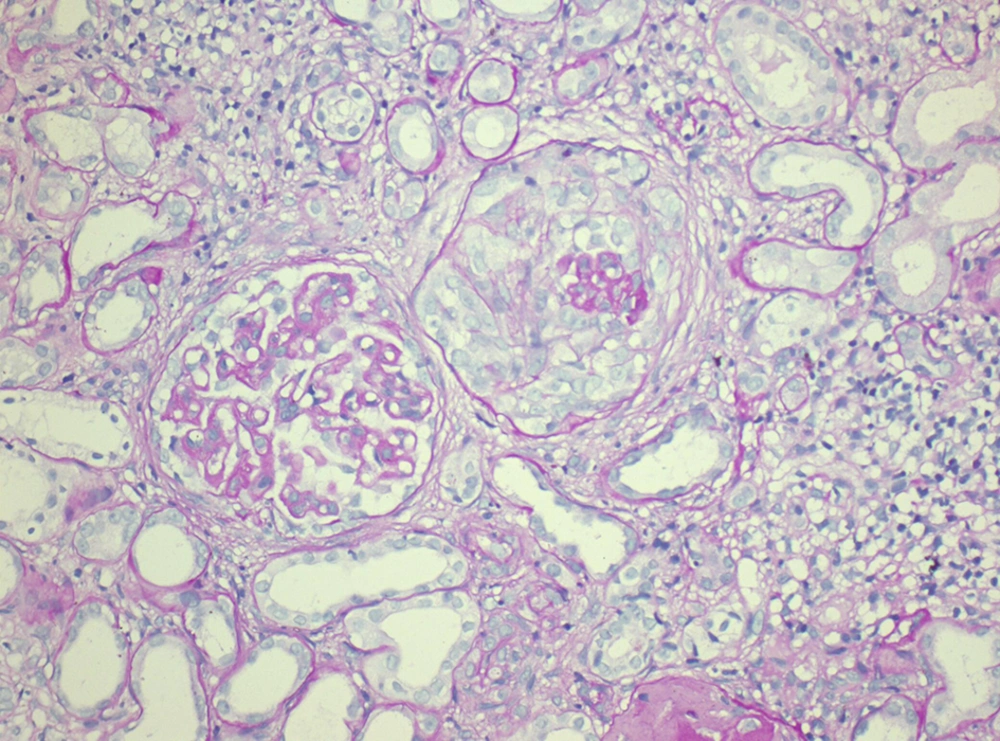
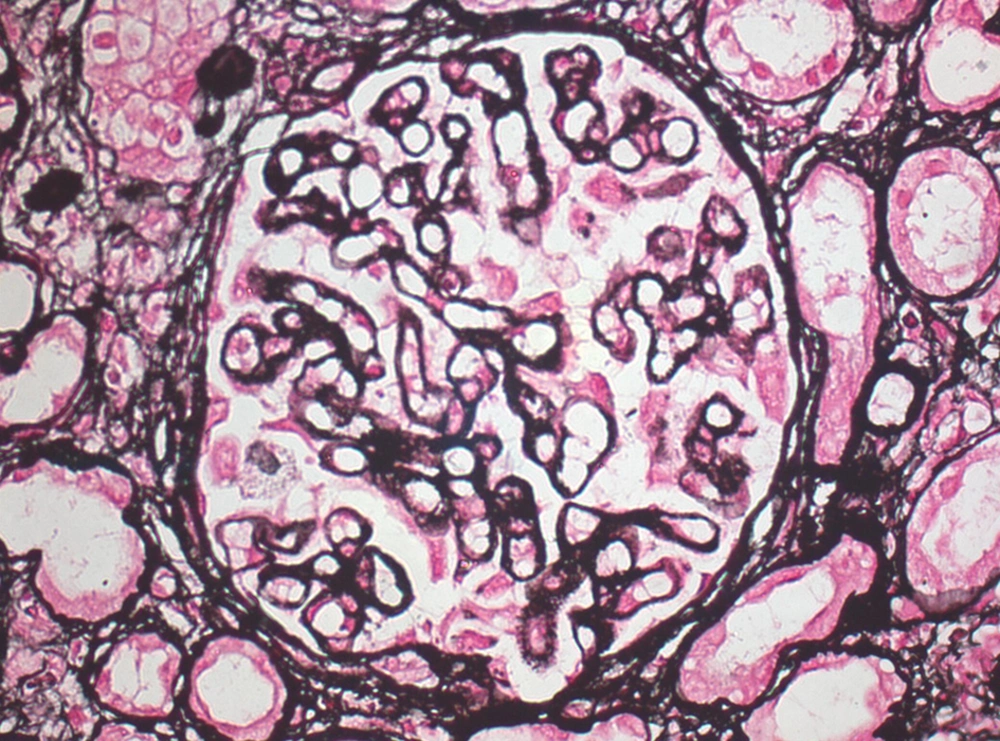
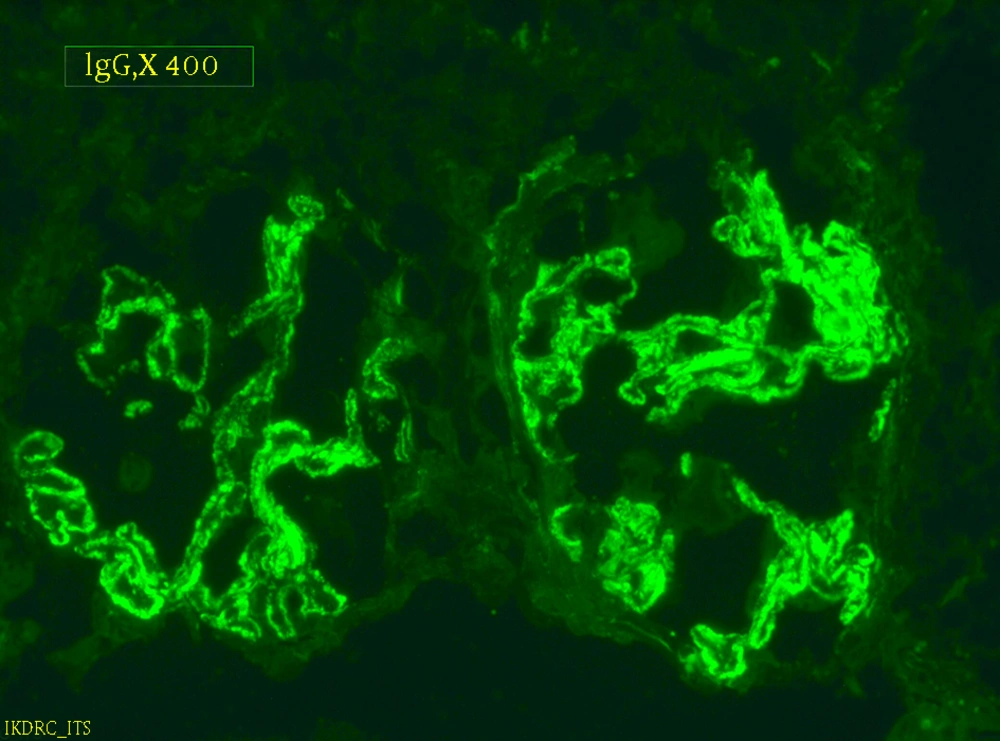
Serum anti-nuclear antibody (ANA), the levels of serum complements C3 and C4 were in normal limits. Chest radiograph revealed normal findings and renal ultrasonography showed right kidney dimension of 8.6 × 3.4 cm and left kidney dimension of 9.0 × 4.5 cm, with increased echogenicity and maintained corticomedullary differentiation. Renal biopsy was performed and after paraffin embedding, 3-µm-thick sections were prepared and stained by hematoxylin and eosin (H and E), periodic acid Schiff, Jone’s silver methenamine, and Gomori’s trichrome stains. Histopathologic examination (Figures 1 and 2) showed a single core of renal tissue containing 14 glomeruli with surrounding tubules and vessels. About eight glomeruli were sclerosed. Remaining viable glomeruli showed mild mesangial prominence. Five glomeruli showed circumferential cellular/fibrocellular crescents. Capillary membranes were thickened with subepithelial spikes. Tubules were moderately atrophied. Interstitium was moderately prominent for focal fibrosis and moderate leucocytes infiltration. Blood vessels were unremarkable. Immunofluorescence (IF) studies (Figure 3) showed fine granular fluorescence (+ 3/4) across 80% to 90% of glomerular capillary walls on staining with anti-human IgG. No fluorescence was revealed on staining with anti-human IgA, C3, C1q, fibrinogen, and IgM antisera. He was diagnosed as a case of MPO-ANCA-associated crescentic GN with MN. He was treated with intravenous methylprednisolone (500 mg/d) for three days, followed by intravenous cyclophosphamide (500 mg) and oral prednisolone (0.5 mg/kg/d) with antihypertensive drugs. He received three units of packed red cells. After two-month follow-up, his serum creatinine was 389 μmol/L, urine albumin was 3 + with 5 to 7/HPF of RBCs. He remained on regular hemodialysis with oral steroid and antihypertensive drugs. His repeated serum MPO-ANCA was 220 U/mL.
3. Discussion
Our patient had positive results for MPO-ANCA with marked proteinuria and hypoalbuminemia. His renal biopsy revealed crescents and thick GBM with subepithelial spikes along with IgG deposition on IF staining. These findings favored diagnosis of MPO-ANCA-associated GN with MN. Coexistence of ANCA-GN with MN is very rare (1, 4-7). Hanamura et al. reported six patients with MPO-ANCA-associated GN with MN (8). Surindran et al. detected coexistence of anti-phospholipase A2 receptor antibody (PLA2R)-positive MN and ANCA-GN (9). Ram et al. reported MN superimposed on Churg-Strauss syndrome (10). The combination of anti GBM-GN and MN are also reported (11, 12). Nasr et al. and Nayak et al. reported that MN preceded the development of anti GBM in 50% of cases (1, 13). Our patient was diagnosed as MPO-ANCA-associated GN with MN simultaneously. Morizane et al. have reported a case of MPO-ANCA-associated GN, which was superimposed on type-3 MPGN. They found subepithelial and subendothelial deposit favoring type-3 MPGN (14).
MN can be classified into primary and secondary forms according to IgG subclass. In primary MN, IgG4 is positive and in secondary MN, IgG1 and igG4. Suwabe et al. speculated that MPO-ANCA-associated GN might cause secondary MN as both IgG1 and igG4 were present in their case (4). The membranous lupus nephritis (LN) with focal or diffuse LN may have crescents with MN. Such mixed patterns of LN have endocapillary proliferation and concomitant subendothelial immune deposits with full-house staining by IF. Our patient did not reveal these features and he had negative serologic lupus markers. The etiopathogenesis of MN and ANCA-associated GN are distinct. Nasr et al. reviewed kidney biopsies from January 2000 to February 2008 and concluded that coexistence of MN and ANCA-associated GN would be coincidental (1). The primary MN is associated with HLA-DQ1 and PLA2R, suggesting genetic predisposition (9, 15). ANCA-associated GN is also associated with environmental triggers such as drugs and infections (9).
Coexistence of MN with MPO-ANCA crescentic GN is very rare. When patient presents as rapidly progressive glomerulonephritis with marked proteinuria, further investigations to rule out possibility of MN with ANCA associated crescentic GN should be performed and the patient should be managed aggressively.