1. Background
Cis-diamminedichloroplatinum II or cisplatin (CP), an important antineoplastic drug, is widely used for treatment of solid tumors including head and neck, lung, testis, ovary, and breast tumors (1). Unfortunately, it has some side effects such as ototoxicity, gastrotoxicity, myelosuppression, and allergic reactions. The major side effect of CP is nephrotoxicity (2), which occurs in about 30% of patients treated with CP due to tubular toxicity, inflammation, oxidative stress, and change in the renal blood flow (3-6). Various agents such as vitamins C and E, losartan, L-arginine, magnesium, and erythropoietin have been proposed as nephroprotective agents against CP-induced nephrotoxicity (7-14). Previous studies showed that CP-induced nephrotoxicity is sex-related and intensity of kidney damage in males treated with CP is significantly greater than that in females (15, 16). At first sight, it seems that the sex differences in CP-induced nephrotoxicity must be related to sex hormones; however, according to our previous study, estrogen did not have protective effect against CP-induced nephrotoxicity and might promote toxicity in female rats (17). The role of testosterone (TS) against CP-induced nephrotoxicity is not yet documented. It seems that TS affects CP effectiveness in proximal tubule with vasodilatory effect on renal afferent arterioles via expression of androgenic receptors and activation of nitric oxide synthase (NOS) (18). Previously, it was reported that TS enhances kidney susceptibility to ischemia-reperfusion injury and on the other hand, it had protective role in ischemia-reperfusion-induced acute kidney injury (19, 20). We hypothesized that TS might affect CP-induced nephrotoxicity,
2. Objectives
This study aimed to examine the role of different doses of TS in response to CP-induced nephrotoxicity in castrated rats.
3. Materials and Methods
3.1. Animals
The investigation was performed on 54 adult male Wistar rats (Animal Center, Isfahan University of Medical Sciences, Isfahan, Iran) with the mean weight of 182 ± 2.7 g. The rats were housed at the temperature of 23℃ to 25 ºC with free access to water and chow. The experimental procedures were approved by the Isfahan University of Medical Sciences Ethics Committee.
3.2. Experimental Protocol
The rats were anesthetized with intraperitoneal injection of ketamine (75 mg/kg). A midline incision of about 2-cm length was made in the subabdominal region. The epididymis and testis were pulled out, vas deferens and spermatic blood vessels were ligated, and finally, testes were removed. One week later, they were randomly allocated to eight experimental groups. Groups 1, 2, and 3 received 10, 50, and 100 mg/kg/wk intramuscular TS enanthate (Aburaihan Co., Tehran, Iran), respectively, for four weeks and at the end of the third week, the animals received 2.5-mg/kg/d CP intraperitoneally for seven days. Group 4 (positive control) received CP plus sesame oil instead of TS. Groups 5, 6, and 7 received the same treatment regimen as groups 1 through 3, respectively, for four weeks; however, they received saline instead of CP for the following seven days. Group 8 (negative control) received sesame oil and saline instead of TS and CP, respectively. When CP injection was initiated, the animals were weighed daily. At the end of the study, blood samples were obtained and then the animals were sacrificed. Left and right kidneys were removed and weighed immediately for histopathologic investigation and measurements.
3.3. Measurements
The serum creatinine (Cr) and blood urea nitrogen (BUN) levels were determined using quantitative diagnostic kits (Pars Azmoon, Iran). The serum and kidney levels of nitrite (stable NO metabolite) were measured using a colorimetric ELISA kit (Promega Corporation, USA) that involved the Griess reaction. The serum and kidney levels of malondialdehyde (MDA) were determined by 10% trichloroacetic acid (TCA) and 0.67% thiobarbituric acid (TBA). TCA test is based on protein deposition. MDA reacted with TBA and created pink complex. The serum level of TS was measured using enzyme immunoassay ELISA kit (Diagnostics Biochem Canada Inc., Canada).
3.4. Histopathological Procedures
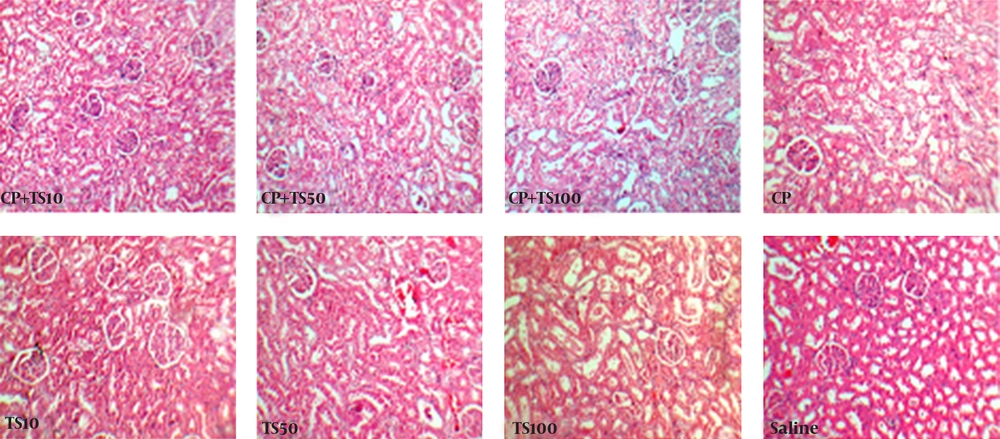
The left kidney was fixed in 10% neutral formalin solution and embedded in paraffin for hematoxylin and eosin staining to examine the tubular damage. A pathologist who was completely unaware of the study protocol and administered medications evaluated the damage. Kidney tissue damage score (KTDS) was graded from I to IV, based on the intensity of tubular lesions (hyaline cast, debris, vacuolization, flattening and degeneration of tubular cells, and dilatation of tubular lumen), while zero was assigned to normal tubules without any damage.
3.5. Statistical Analysis
Data were expressed as mean ± standard error of means (SEM). Comparison of the groups with regard to the bodyweight (BW) loss, kidney weight (KW); and levels of BUN, Cr, MDA, TS, and nitrite were performed by one-way ANOVA followed by post hoc Dunnett's test when applicable. Comparison between positive and negative control grous was performed by t-Student test. The histopathologic damage score of the groups was compared by the Kruskal-Wallis and Mann-Whitney U tests.
4. Results
4.1. Effect of Cisplatin on Renal Toxicity
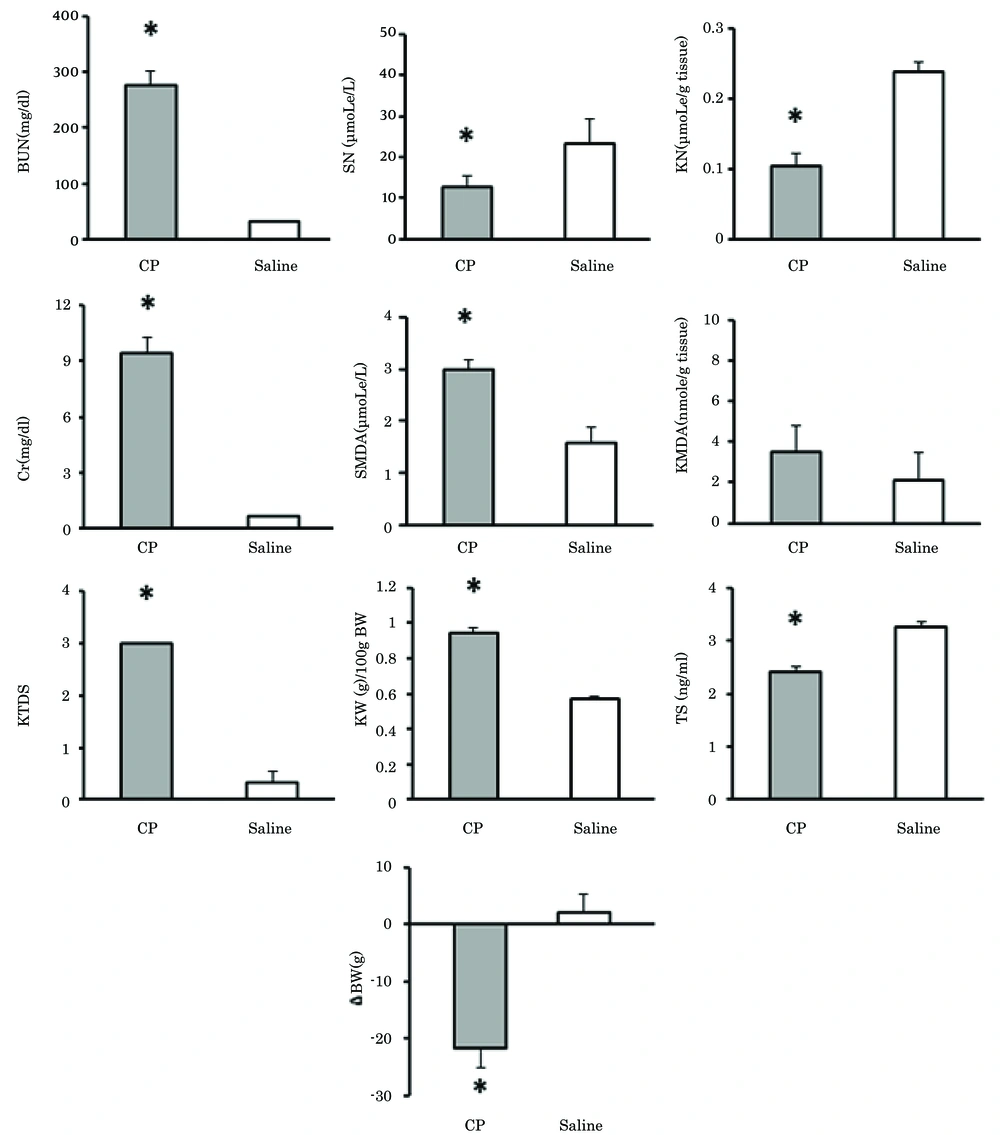
Comparison of the positive and negative control groups (group 4 and group 8, respectively) demonstrated that in the positive control group, KW, KTDS, and serum levels of BUN, Cr, and MDA had increased while the serum TS level, serum and kidney tissue levels of nitrite, and BW had decreased significantly (P < 0.05). These data confirmed CP-induced nephrotoxicity (Figure 1).
Significant difference between positive and negative control groups (P < 0.05). Abbreviations: BUN, blood urea nitrogen; Cr, creatinine; SN, serum nitrite; SMDA, serum malondialdehyde; TS, testosterone; KN, kidney tissue levels of nitrite; KMDA, kidney tissue levels of malondialdehyde; KW, kidney weight; ΔBW, bodyweight changes; and KTDS, kidney tissue damage score.
4.2. Effect of Testosterone on Cisplatin-Induced Nephrotoxicity
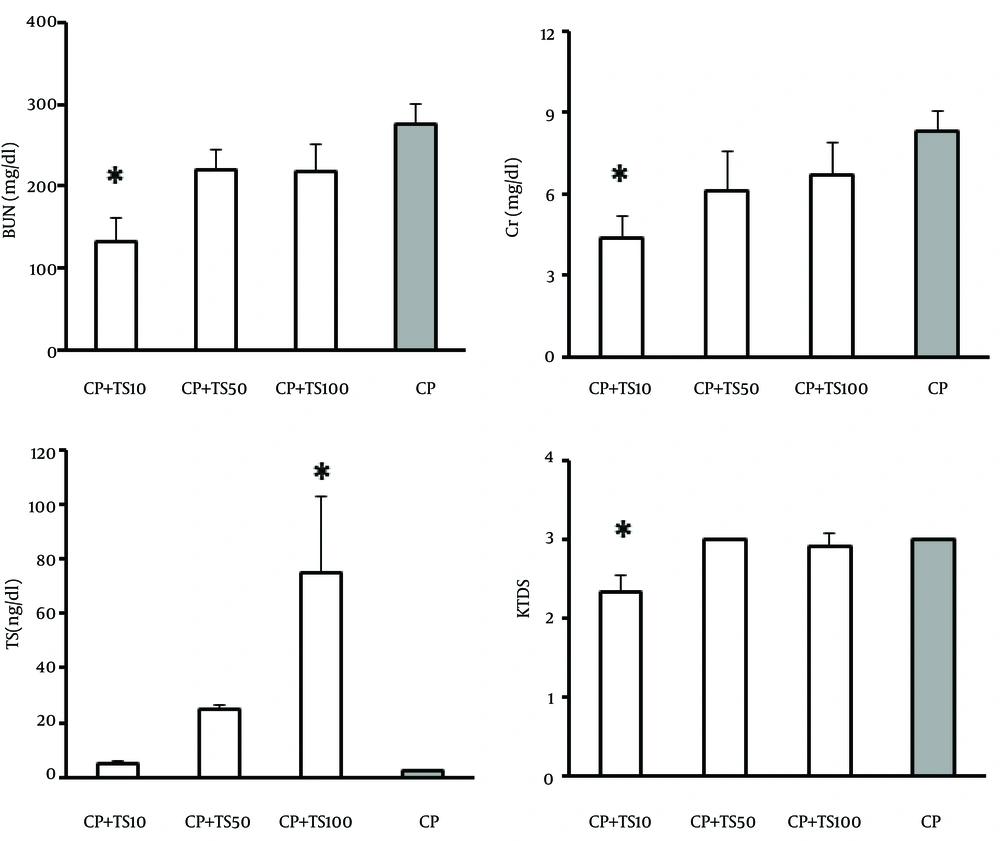
Comparison between the case groups (groups 1, 2, and 3) and the positive control (group 4) indicated that the serum levels of BUN and Cr, and KTDS had significantly decreased in group 1 (group treated with low-dose TS plus CP) in comparison with group 4 (positive control group) (P < 0.05) (Figure 2). According to these findings, low-dose TS can prevent CP-induced nephrotoxicity. As expected, significant increase in serum TS level was detected in group 3 (group treated with high-dose TS) in comparison with the positive control group (P < 0.05) (Figure 2). Although the serum levels of TS in groups 1 and 2 were higher than that in group 4, these differences were not statistically significant (Figure 2). No significant differences were observed among the groups with respect to other parameters (Table 1).
| Group c | SN, µmoL/L | KN, µmoL/g tissue | SMDA, µmoL/L | KMDA, nmole/g tissue | KW, g/100g BW | ΔBW, g |
|---|---|---|---|---|---|---|
| 1. CP + TS10 | 14.69 ± 2.224 | 0.10 ± 0.01 | 2.71 ± 0.32 | 1.14 ± 0.32 | 0.97 ± 0.04 | -15.33 ± 2.80 |
| 2. CP + TS50 | 21.39 ± 5.71 | 0.13 ± 0.02 | 3.08 ± 0.46 | 1.77 ± 0.52 | 0.89 ± 0.04 | -15.28 ± 4.14 |
| 3. CP + TS100 | 17.81 ± 4.07 | 0.13 ± 0.01 | 2.56 ± 0.35 | 5.91 ± 2.66 | 0.92 ± 0.04 | -15.9 ± 2.27 |
| 4. CP | 12.78 ± 2.56 | 0.1 ± 0.01 | 2.98 ± 0.19 | 3.54 ± 1.23 | 0.94 ± 0.03 | -21.83 ± 3.23 |
aAbbreviations: CP, cisplatin; TS, testosterone; SN, serum nitrite; KN, kidney nitrite; SMDA, serum malondialdehyde; KMDA, kidney tissue malondialdehyde; KW, kidney weight; and ∆BW, bodyweight changes.
bNo significant differences were observed between these groups.
cTS10, TS50, and TS100 stand for treatment with testosterone doses of 10, 50, and 100 mg/kg/wk, respectively.
4.3. Effect of Testosterone on Renal Toxicity
Comparison between TS alone treated groups (groups 5, 6, and 7) in one hand and negative control group (group 8) on the other hand indicated no significant differences in serum levels of BUN, Cr, and nitrite as well as tissue levels of nitrite and MDA (Table 2). Nevertheless, increase in serum MDA, KW, and KTDS were observed in high-dose TS (100 mg/kg/wk) group (P < 0.05). The BW reduced in TS treated groups and this reduction was significant only in group 7 in comparison with group 8 (P < 0.05). The serum level of TS was significantly higher in groups 6 and 7 (P < 0.05). The samples of tissue images for all groups are demonstrated in Figure 3.
| Group c | BUN, mg/dL | Cr, mg/dL | SN, µmoL/L | KN, µmoL/g tissue | SMDA, µmoL/L | KMDA, nmoL/g | KTDS | KW, g | ΔBW, g | TS, ng/mL |
|---|---|---|---|---|---|---|---|---|---|---|
| 5. TS10 | 34.42 ± 3.355 | 0.72 ± 0.05 | 27.63 ± 5.53 | 0.22 ± 0.02 | 2.77 ± 0.48 | 1.51 ± 0.73 | 0 ± 0 | 0.71 ± 0.03 | -1.66 ± 1.38 | 8.67 ± 3.77 |
| 6. TS50 | 26.66 ± 3.75 | 0.87 ± 0.1 | 18.11 ± 6.07 | 0.22 ± 0.01 | 2.64 ± 0.52 | 0.89 ± 0.31 | 0.67 ± 0.21 | 0.68 ± 0.04 | -3.83 ± 1.32 | 61.12 ± 0.54 b |
| 7. TS100 | 41.83 ± 5.79 | 0.86 ± 0.24 | 22.1 ± 6.59 | 0.17 ± 0.01 | 3.37 ± 0.23 b | 1.45 ± 0.49 | 1.5 ± 0.22 b | 0.94 ± 0.08 b | -5.75 ± 1.6 b | 89.85 ± 6.08 b |
| 8. Saline | 32.08 ± 1.97 | 0.7 ± 0.02 | 23.16 ± 6.001 | 0.23 ± 0.01 | 1.59 ± 0.29 | 2.12 ± 1.34 | 0.33 ± 0.21 | 0.57 ± 0.01 | 2 ± 3.15 | 3.25 ± 0.11 |
a Abbreviations: CP, cisplatin; TS, testosterone; BUN, blood urea nitrogen; Cr, creatinine; SN, serum nitrite; KN, kidney tissue nitrite; SMDA, serum malondialdehyde; KMDA, kidney tissue malondialdehyde; KW, kidney weight; KTDS, kidney tissue damage score; and ∆BW, bodyweight changes.
bSignificant difference from group 8 (P < 0.05).
c TS10, TS50, and TS100 stand for treatment with testosterone doses of 10,50, and 100 mg/kg/wk, respectively.
5. Discussion
The major finding of the present study was protective role of low-dose TS against CP-induced nephrotoxicity in castrated rats. Previous studies identified the role of estrogen in CP-induced nephrotoxicity (17), but the role of TS in CP-induced nephrotoxicity has not been well-documented. The effect of CP on serum levels of BUN, Cr, and MDA, kidney damage, KW, and BW loss were reported (11, 21-28). In addition, decrease in the serum levels of nitrite and TS with CP has been demonstrated (29-31). The present study confirmed these findings. Despite orchiectomy and elimination of TS source, the serum level of remaining TS significantly decreased in group 4 in comparison with group 8. This suggests that CP might cause a depression in serum TS level (31). In our study, the serum levels of BUN and Cr and kidney damage were decreased in low dose of TS plus CP treated group (group 1) in comparison with the positive control group, which revealed the protective role of TS. It seems that TS at high doses did not have any protective role against CP-induced nephrotoxicity and might acts as a toxic agent. In addition, we found that TS alone at high doses had raised serum level of MDA, KW, and KTDS while it had reduced BW significantly. Some researches demonstrated that in ischemia-reperfusion model, the serum level of Cr and proximal tubule injury might decrease in castrated rats that had received TS. Therefore, TS might protect the kidney from ischemia-reperfusion-induced acute kidney injury due to fast vasodilation effect on renal afferent arterioles, which is mediated by androgen receptors and activation of nitric oxide synthase due to an acute increase in glomerular filtration rate (18, 20). In addition, chronic diseases like obesity, hypertension, cardiovascular diseases, and chronic kidney disease (CKD) are associated with deficiency of TS in males (32-36). Reduced TS levels in men with nondialysis CKD and in males on hemodialysis have been reported and TS deficiency aggravates cardiovascular events and increases arterial stiffness and mortality (37, 38). Furthermore, hypogonadism is a cause of anemia in men with CKD and TS replacement therapy decreases the prevalence of anemia in these patients (39). In the current study, the high dose of TS in TS alone treated rats (group 7) increased MDA level and KTDS. According to some studies, TS increases plasma MDA, as an oxidative stress parameter, and Cr. TS elevates kidney susceptibility to ischemia-reperfusion injury through decreased activation of NOS and increased oxidative stress. It also promotes susceptibility of proximal tubule cells to apoptotic damage (19, 40, 41). On the other hand, high dose of TS enhances oxidative stress and counteract antioxidant defense of myocardium (42). In the present study, high dose of TS decreased animal BW, which was similar to reported observation in human where TS replacement therapy in hypogonadal men led to loss of BW (43).
Our results suggest that TS in low doses plays a protective role against CP-induced nephrotoxicity and in high dose has a promoting role in nephrotoxicity; however, TS does not possess antioxidant properties at pharmacologic concentrations (44). It can be concluded that CP therapy should be avoided when serum TS level is high because TS in high concentrations promotes CP-induced nephrotoxicity.