1. Introduction
Patients with end-stage renal disease (ESRD) experience sleep disorders more frequently than normal population do and despite the great influence of sleep disturbances on their quality of life, it remains unrecognized by many renal healthcare providers. About 50% of patients with ESRD are affected by at least one of the sleep disorders including insomnia, sleep apnea, excessive daytime sleepiness, restless legs syndrome (RLS), and periodic limb movement disorder (1, 2).
Patients with ESRD who are affected by sleep disorders are more at risk of cardiovascular events and ventricular remodeling, high blood pressure, immunosuppression, and infections, which cannot be justified by sleep disorder alone or sleep deficit side effects that could leads to fatigue, anxiety, and depression (3). The mortality rate in patients on hemodialysis (HD) with RLS is higher than in those without this condition (4).
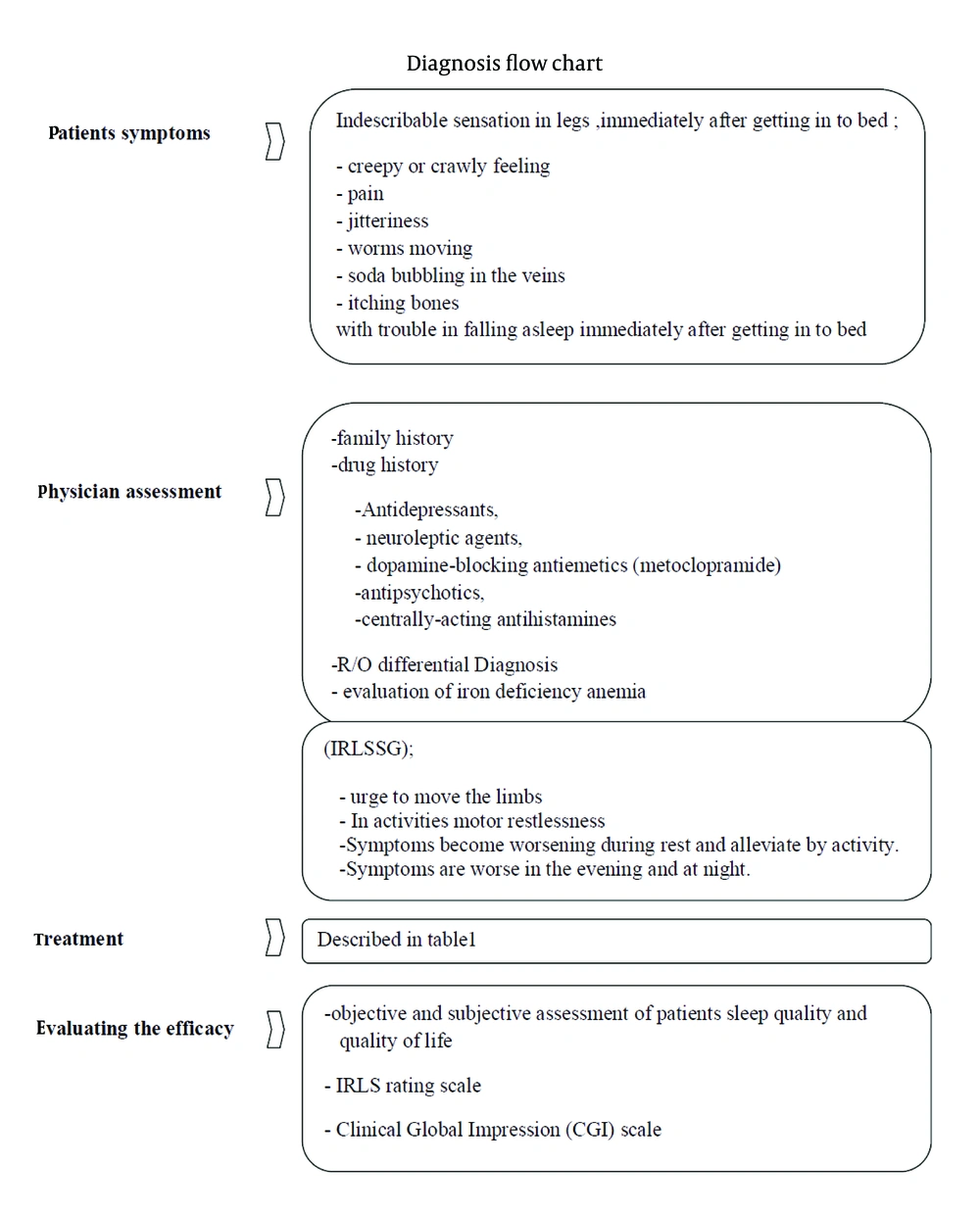
RLS is an irresistible urge to move legs that usually occur during inactivity or at rest and becomes worse at evening and night. In 1945, Ekbom (5) has introduced RLS to medical literature; however, Sir Tomas Willis had described the symptoms 300 years earlier (6). Due to the significant influence of RLS symptoms on sleep quality, it can be a disabling condition. On the other hand, symptoms are usually indescribable. Patients who are affected by RLS find it difficult to explain or sometimes they use funny explanation like creepy or crawly feeling or sometimes they explain it as pain, jitteriness, worms moving, soda bubbling in the veins, and itching bones (7), which makes the diagnosis difficult for clinician and it becomes missed or underdiagnosed despite being simply curable. In the sever form of the disease this sensation in legs could also involve other body parts like hips, trunk, hands, or even face but symptoms are more severe in legs, which are the first affected part (7).
Immediately after getting into bed, patients have trouble in falling asleep (onset of sleep) or difficulties for getting back to sleep (maintaining sleep). Symptoms are often improved by walking or pacing the floor; therefore, they are usually awake and walking away or moving in the bed until midnight. Sleep disruptions could lead to daytime sleepiness and fatigue (8). RLS is a lifelong disorder and although it could have a fluctuating course, permanent remission is rare (9). RLS can occur as a primary or secondary disorder. The secondary type is usually due to iron deficiency anemia, pregnancy, or renal failure. Some studies have reported that diabetes mellitus, Parkinson’s disease, or different forms of neuropathy are associated with RLS.
Generally, two different phenotypes have been defined with respect to the patient’s age at onset of symptom, namely, early-onset and late-onset RLS. In early-onset RLS or primary form, symptoms usually develop before 45 years of age and progress slowly; patients have positive family history and RLS is common in the first-degree relatives. In the late-onset RLS (usually secondary form), patients more often have comorbid disorders, symptoms progressive rapidly, and neuropathies are more common (9-11). Despite the high prevalence of RLS in patients on HD and its enormous influence on their sleep as well as life quality, it can be treated easily. Physicians have to be mindful of the RLS diagnosis, which depends on clinical interview as well as clinician experience and patients’ expression.
2. Epidemiology and Risk Factors
RLS symptoms have been reported in 2.5% to 15% of the general population and it is estimated that 20% to 30% of patients with uremia are affected by RLS. Differences in the estimated prevalence rate in different studies might be due to not using the full criteria of International RLS Study Group (IRLSSG). Moreover, other underlying factors such as the technical nature of the diagnosis, which is based on interview and patient’s history, small population of the studies, and lack of attention to RLS in the differential diagnosis might lead to neglect the diagnosis. On the other hand, different studies have reached to almost the same prevalence by using the same set for diagnostic criteria and it seems that the different reported prevalence rates have no association with the racial or ethnic differences (10).
Some studies have shown that RLS is more prevalent in females. Berger et al. reported that RLS affected females ten times as more as males; they also showed that the prevalence of RLS in young nulliparous women and in men had no significant difference and proposed parity as a major risk factor for RLS (12). In addition, the role of estrogen excess in women (e.g. pregnancy-related RLS) or iron deficiency should be noted. Some other risk factors such as old age and drugs consumption including dopamine antagonists, tricyclic antidepressants, and serotonin reuptake inhibitors, excessive caffeine or alcohol intake, and nicotine may exacerbate symptoms (4, 13). According to a cross-sectional study by Bastos et al. (14) on 100 patients on HD, RLS symptoms are not associated with dialysis sessions and renal function parameters, which was similar to the findings of Araujo et al. (15) study on 400 patients on HD; moreover, there was no association between degree of parathormone levels or uremia with uremic RLS (16).
3. Pathophysiology
Although RLS has been described for more than sixth decade, pathophysiology of the RLS is still unclear. In this regard, there are two major theories: iron deficiency and central nervous system (CNS) dopamine imbalance
First-degree relatives of a patient with RLS are seven times more at risk of being affected by RLS than general population is; in addition, some studies have reported the association of genetic factors (chromosomal changes 12q22-23, 14q13-21, and 9p24-22) with RLS. It seems that some interactions between genes, gene modifiers, and environmental factors are involved.
3.1. Iron Deficiency
The association between iron deficiency and RLS has been suggested for many years, which was derived from reports of some studies about a negative correlation between patients’ serum ferritin levels and RLS symptoms; in addition, association of iron deficiency state with secondary forms of RLS (i.e. pregnancy and ESRD-associated RLS) has been explained. The theory of insufficient brain iron status had been suggested after the results of some studies on CSF, brain imaging, ultrasonography, and autopsy showed decreased ferritin and iron storage in neuromelanin cells of RLS patients, decreased CSF ferritin and prohepcidin (regulatory protein for intracellular iron), and elevated levels of transferrin.
We have to mention that peripheral blood iron and ferritin values are not good predictors for intracellular iron levels, which has an important role in enzymatic functions. Moreover, the ferritin is an acute phase reactant protein and a high ferritin level in patients with ESRD is not a reliable measure of iron status (17). In their valuable research, Conner et al. described that the endothelial cells of the blood-brain barrier act as an iron reservoir for brain cells. Therefore, lack of sufficient reserved iron or regulatory mechanisms could have an important role in RLS and associated conditions such as physiologic challenges (e.g. pregnancy), dysregulation of transferrin receptors, and decreased CNS ferritin levels (18). In another study, Sloand et al. showed that the high-dose iron dextran infusion in patients with ESRD and adding iron supplements in some patients with anemia could refill the reservoir and reduce the symptoms severity (17).
3.2. CNS Dopamine Imbalances
Clinical observations support a central role for dopamine in RLS. Patients’ symptoms had dramatically improved by dopamine agonist (DA) (even in low doses and from the first dose) and became worsened or elicited with dopamine antagonists (19, 20). Neuroimaging and positron emission tomography (PET) studies have shown increased dopamine (D2) levels in striatum and extra-striatal regions, which is related to receptors upregulation or decreased dopamine levels. Moreover, we have to mention the importance of reported association between Parkinson’s disease and RLS in some studies (10).
Due to suppression of thyroid axis by DA, circadian rhythm of TSH secretion, and decrease in the level of TSH as well as the severity of RLS symptoms in the evening, Pereira et al. explained a hypothesis of the role of imbalance between dopaminergic system and thyroid hormones (TH) in RLS symptoms. They also noted that iron deficiency with decreased cytochrome P450 (CYP450) enzymes, which is needed for degrading TH, and reduction of TH could induce the RLS symptoms (21). So far, many studies reported no association between RLS symptoms and renal function markers. The pathogenesis of RLS in patients with ESRD is unclear yet. Although the role of iron abnormalities in uremic RLS was suggested, extensive use of high-dose intravenous (IV) iron and erythropoietin on dialysis units weakens the existence of such an association (22).
4. Diagnosis
The four cardinal diagnostic features of IRLSSG diagnostic criteria include:
1. A convincing urge to move the limbs, which is generally associated with dysesthesia or paresthesias.
2. Inactivity motor restlessness such as tossing or turning in bed, ground pacing, or leg rubbing.
3. Symptoms develop or are aggravated during rest and alleviated by activity.
4. Symptoms are worse in the evening and at night.
It is important to note that all four parts are necessary for diagnosis. Sleep disturbances and daytime fatigue could develop in association with RLS. Patients with primary RLS have normal findings in neurologic examination and may experience spontaneous, rhythmic, episodic limb movements in sleep or while awake and at rest (Figure 1).
5. Differential Diagnosis
Numerous disorders can be considered in differential diagnosis of RLS. Therefore, clinician should be attentive to the conditions with similar symptoms. Peripheral nervous system disorders such as polyneuropathies or radiculopathies have sensory neural symptoms such as pain or paresthesia that can be similar to RLS; however, they would not alleviate by moving the limb. Other sleep disorders and some rare conditions in the differential diagnosis including vascular disorders such as restless red legs or burning feet syndrome, painful legs and moving toes, and hypotensive akathisia in patients with autonomic failure or drug-induced akathisia have to be considered in differential diagnosis (9, 13, 23, 24).
6. Treatment
Pharmacologic and nonpharmacologic therapies are used in RLS (Table 1).
| Level of Disorder (Medication) | Level of Recommendation |
|---|---|
| Mild to Intermittent | |
| Dopamine Agonists | Standard level |
| Levodopa | Guideline level |
| Benzodiazepine | Option level |
| Low-Potent Opioid | Guideline level (but off-label use) |
| Disabling and Persistent | |
| Dopamine Agonist | |
| Alpha-2-Delta Calcium Channel Ligands | |
| Gabapentin | Guideline level |
| Gabapentin Enacarbil | |
| Pregabalin | |
| Refractory | |
| Dopamine Agonists | |
| Levodopa | |
| Gabapentin, Gabapentin Enacarbil | |
| Opioid (Oxycodone, Hydrocodone, and Methadone) | |
| Clonidine | Option level |
| Carbamazepine | Option level |
| Propranolol | Option level |
| Uremic Restless Leg Syndrome | |
| Gabapentin | |
| Ropinirole | |
| Pramipexole | |
| Levodopa | |
| Benzodiazepines | |
| Intravenous Iron Dextran | |
| Additional Therapies | |
| Nonpharmacologic Therapies | |
| Iron Therapy If Ferritin Level < 75 μg/L |
Treatment of Restless Leg Syndrome
6.1. Nonpharmacologic Treatment
These nonpharmacologic methods are often use in mild to moderate forms of RLS and modifying drug usage are considered for severe forms:
1. Mental alerting actions at times of inactivity
2. Avoidance of aggravating factors:
a. Antidepressants, neuroleptic agents, dopamine-blocking antiemetics (metoclopramide), antipsychotics, or centrally acting antihistamines may contribute to initiation of RLS or worsening of previous symptoms.
b. Alcohol, nicotine, or caffeine
3. Moderate standard exercise (25)
4. Pneumatic compression and leg message (26)
5. Appling heat by hot baths or heating pads
6. Short daily HD for patients with uremic RLS
7. Dissolving concerns before bedtime and not forcing to sleep (27)
8. A regular sleep schedule and avoiding daily naps (28).
6.2. Pharmacologic Treatment
Common pharmacological agents for the treatment of RLS in clinical practice are DAs, levodopa, Alpha-2-delta calcium channel ligands, opioids, anticonvulsants, benzodiazepines, or drugs acting on adrenergic systems such as clonidine (24). In patients with mild or intermittent RLS in which symptoms are not develop daily but are disabling when they are presented, daily treatment is not necessary. We could apply intermittent use of DAs, levodopa (25 mg, one-half or one tablet at bedtime), a benzodiazepine (clonazepam, 1-2 mg daily or diazepam) especially in younger patients, or a low-potent opioid and nonpharmacologic therapies (29). The clinicians should be cautious about the early morning rebound of symptoms or daytime RLS augmentation by levodopa (24, 30). If patients experience disabling and persistent daily symptoms, a DA or an alpha-2-delta calcium channel ligand would be the first line of treatment in addition to nonpharmacologic therapy and iron deficiency treatment (31).
6.2.1. Dopamine Agonist
Non-ergot DAs such as pramipexole (0.125 mg once a day; the best therapeutic dose, 0.25 mg/day) and ropinirole (initiating with 0.25 mg once a day and increasing up to 4 mg until the symptoms relieve) have standard level of recommendation with fewer side effects such as lightheadedness, nausea, and fatigue, which will improve over the time.
6.2.2. Alpha-2-Delta Calcium Channel Ligand
Alpha-2-delta calcium channel ligands can be used as an alternative to DAs, especially in patients who had an underlying disorder such as neuropathy, insomnia, or other sleep disturbances. These drugs include gabapentin (200-300 mg after HD, three times a week), gabapentin enacarbil (600 mg, in the morning), and pregabalin (300 mg/day). According to the risks of heart valve damage, clinicians should be cautious about using pergolide routinely (31, 32). Treatment is applied for six to 12 months and patients should be evaluated for drug side effects such as somnolence, dizziness, headache, and fatigue (29).
Refractory RLS is a form of disease that the patient symptoms do not improve despite the full dose of daily treatment with a DA or levodopa. Changing DA drug or adding the second agent such as gabapentin enacarbil, gabapentin, or a high-potency opioid (30-60 mg of Codeine, 50-100 mg of tramadol, 5-15 mg of oxycodone, 5-15 mg of hydrocodone, and 5-10 mg of methadone) is suggested (33). Despite the risk of dependency, opioids are approved (but off-label use) in RLS (24). In addition, other drugs such as clonidine (0.05 mg/day), carbamazepine (mean dose, 236 mg/day), and propranolol (40-120 mg/day) are suggested with an option level of recommendation (24).
6.3. Uremic Restless Leg Syndrome
In patients with ESRD, the clinician should be cautious about the drug side effects and make judgment about the benefits-harm balance of treatment. Some randomized clinical trials have shown that gabapentin, ropinirole, pramipexole, levodopa, clonidine benzodiazepines (clonazepam), IV iron dextran, and erythropoietin during dialysis and exercise can improve symptom of uremic RLS (17, 24). Kidney transplantation could resolve RLS symptoms but risk of mortality does not change (16, 33).
6.4. Iron Therapy
If patients serum ferritin level is lower than 100 μg/L, oral or IV iron supplement should be administered. Although the IV iron improves patients iron status more quickly, in practice, we have to mention its expensive cost and risk of anaphylactic reaction. We can use the oral form as ferrous sulfate (325 mg, two to three times a day) with vitamin C supplement; using other supplements such as calcium can decrease iron absorption. After three to four months of iron therapy, we should evaluate the ferritin levels and if the values were still < 75 μg/L and iron saturation was > 20%, the treatment should be continued and patients should be evaluated every three to six month. Iron deficiency anemia in patients with HD should be treated only by IV iron therapy (29).
6.5. Acupuncture
Despite some studies that showed the effectiveness of acupuncture for remission of unpleasant sensations in the legs in combination with medications or massage, there is insufficient evidence and further well-designed, large-scale clinical trials are needed to confirm the effectiveness of dermal needle therapy as a practical and safe treatment for RLS (34).
7. Treatment Efficacy Measures
Intrenational RLS rating scale (validated in 2003) and the Clinical Global Impression (CGI) scale are used for evaluating the efficacy of treatment, subjective or objective assessment of patients sleep quality, and quality of life. IRLS rating scale has ten Questions and shows the severity of symptoms. CGI has three sections including severity of illness, global improvement or change, and efficacy index. The sleep-related parameters by polysomnography (PSG) or autography are the only objective measurement methods (24).
8. Conclusions
RLS is a disabling sleep problem in affected patients. Although it is a simple-to-alleviate disorder, uncomplicated, and curable condition, lack of attention by healthcare providers makes it a great difficulty for patients, especially in patients with ESRD who are affected by many other morbidities as well as sleep disorders and might deteriorate the quality of their life. Due to difficulty of expressing the symptoms by patients, we have to be cautious about this diagnosis. After evaluating the patients medications and if possible, reducing risk factors, applying sleep hygiene, using DAs or other drugs, and correction of iron profile seems to have a great influence on this condition in patients with ESRD.