1. Background
About 40% of CKD patients have cardiovascular disease (CVD) even before they reach end-stage renal disease (ESRD) (1). Traditional risk factors such as smoking, hypertension, diabetes mellitus, and dyslipidemia, underestimate CVD risk in patients with CKD, including predialysis patients (2). Patients with CKD have nontraditional or uremia-related risk factors, which are responsible for an increased risk of CVD (3). CVD identification in asymptomatic predialysis CKD patients poses a big challenge in their management. Recently, a number of novel cardiovascular biomarkers were identified as predictor of outcomes in patients with CVD (3).
Pro-BNP (108 amino acids) is a prohormone, which is secreted from left ventricle in response to increased myocardial wall stress (4). On release into the circulation, proBNP is broken into two equal proportions: BNP and NT-proBNP. The half-life of BNP is 20 minutes, whereas the half-life of NT-proBNP is 120 minutes (5). That is why the circulating NT-proBNP level is approximately six-fold higher than that of BNP. Cardiac troponin-T (cTnT) is raised in a portion of patients with CKD and ESRD. The reasons for this phenomenon remain unclear. Possible mechanisms include coronary artery disease, left ventricular dysfunction, left ventricular hypertrophy, cardiac micro infarctions, and arrhythmia (6, 7). Nonischemic myocardial injury due to uremic toxins may be re-expression of fetal troponin-T in skeletal muscle myopathy (7). The Food and Drug Administration recently approved the use of cTnT as a biomarker for mortality risk stratification in ESRD, also the use of cTnT for prognostication is recommended by the kidney disease outcomes quality initiative (KDOQI) (8). Inflammation is a very important component of atherosclerosis pathogenesis in CKD patients. In predialysis CKD patients, CRP (C-reactive protein) level increases as GFR (glomerular filtration rate) decreases (9). High-sensitivity CRP (hs-CRP) is recommended to measure cardiovascular risk caused by inflammation (10). Albuminuria is one of the diagnostic criteria for chronic kidney disease. Screening for albuminuria is recommended in patients at increased risk of CKD, including those with hypertension, diabetes, cardiovascular disease, and a family history of CKD (11, 12).
Carotid intima media thickness (IMT) is a noninvasive, sensitive, and specific quantitative measure of subclinical coronary atherosclerosis (13, 14). In prospective studies, carotid IMT predicts clinical cardiovascular events independently of classic risk factors (15, 16). An increment of 0.1 mm in IMT is associated with 24% to 31% increased risk of cardiovascular mortality in dialysis patients (17, 18). If correlation of cardiovascular biomarkers correlates significantly with CIMT, by just measuring levels of biomarkers, we can predict and monitor cardiovascular risk in CKD at an early stage.
2. Objectives
1) To study the prevalence of nontraditional cardiovascular biomarkers and increased carotid intima media thickness (CIMT) in predialysis CKD patients.
2) To study association of NT-proBNP, cTnT, hs-CRP, and spot urine albumin creatinine ratio with carotid intima media thickness (CIMT) for cardiovascular risk estimation in predialysis CKD patients.
3. Patients and Methods
3.1. Study Subjects and Design
The study was cross-sectional one. One hundred and fifty-seven Indian adult patients with CKD at stages 3, 4, and 5 (predialysis) were enrolled prospectively during one-year period (July 2012 to June 2013). Estimated GFR was calculated by using the abbreviated Modification of Diet in Renal Disease (MDRD) study formula (19). According to KDOQI guidelines of the National Kidney Foundation (NKF), CKD stages were divided into stage 3: GFR 30-59, stage 4: GFR 15-29, and stage 5: GFR < 15 (all values of GFR were in mL/min/1.73 m2 body surface area). Excluded from the study were patients receiving dialysis or ESRD, having arteriovenous fistula, suffering from acute renal failure, having functioning transplant, patients with recent (< 1 month) cardiac event, or acute stroke (during last year), patients having clinical evidence of heart failure, symptomatic coronary artery disease, or arrhythmia, and septicemic patients. Eleven patients missed the day of blood sampling. In 9 patients, insufficient plasma or serum for analysis of cardiac biomarkers was obtained. Six patients had a clinical diagnosis of heart failure based on history and physical examination. Four patients showed significant ECG changes. Three patients had arteriovenous fistula. Two patients had stroke during last year. Two patients were found to be septicemic.
In total, 120 predialysis CKD patients (40 patients in each CKD stages of 3, 4, and 5) were analyzed. Patients were treated for CKD by nephrology outpatient department of our hospital. Coronary artery disease (CAD) was defined as having documented old myocardial infarction, coronary artery bypass graft surgery, or percutaneous coronary intervention, and ECG with ischemic changes in apparently asymptomatic patient or presence of angina history. The protocol and consent forms for the study were approved by the Review Board and Ethics Committee of PGIMER Dr RML Hospital, New Delhi, India, and informed consent was obtained from each participant.
3.2. Blood Sampling and Other Analyses
Blood samples were collected and allowed to clot for at least 30 min, then centrifuged at 3000 rpm for 10 min. Samples of plasma were taken for NT-ProBNP measurement. Samples of serum were taken for hs-CRP and cTnT measurement. The resulting serum and plasma were aliquoted, frozen, and maintained at -70°C. Then, samples from all patients were thawed for quantitative measurement of biomarkers. Other procedures were done on the day of sample collection. Quantitative measurement of N-terminal proBNP (NT-proBNP) was done by the Elecsys proBNP immunoassay (Roche Diagnostics, Indianapolis, IN). Cutoffs were 125 pg/mL and 450 pg/mL for age < 75 year and ≥ 75 year, respectively. Quantitative measurement of cardiac troponin T (cTnT) was done by the Elecsys cardiac troponin T assay (Elecsys TroponinT STAT Immunoassay, third generation; Roche Diagnostics). The limit of detection is < 0.01 ng/mL, as given by the manufacturer. The 99th percentile of a reference population is 0.01 ng/mL and the threshold for diagnosis of myocardial infarction in 0.1 ng/mL. Serum hs-CRP was measured by immunoturbidimetry method. The spot Urine albumin to creatinine ratio (ACR) measurement was done on fully automated analyzer by dry chemistry method. The spot urine ACR was considered high when it was ≥ 30 mg/g of creatinine in a single spot urine sample. Serum creatinine was determined using the Autopak reagent kit. This kit utilizes the picrate method.
3.3. Carotid Intima Media Thickness Measurement
The carotid intima media thickness (CIMT) was measured by a high resolution B mode USG system (HDI 1500) having an electronic linear, high frequency broadband transducer with a mid-frequency of 7.5 MHz. Only one operator did the CIMT measurement for all patients. The CIMT was measured at several areas, including the posterior aspect of the common carotid artery, common carotid artery bifurcation, and anterior wall of internal carotid artery. The intima media thickness (IMT) was defined as the distance between the leading edges of the lumen interface and the media-adventitia interface at the far wall. The maximum value of IMT was taken into consideration. The final value of the CIMT was calculated as an average of the maximum IMT on both sides. CIMT was not measured at the site of the atheromatous plaques. The presence of increased CIMT in the carotid artery was defined as a CIMT of > 0.75 mm (20).
3.4. Statistical Analyses
Data were analyzed by statistical package for social sciences (SPSS), version 19 for windows 7. The data were presented as mean ± standard deviation or frequency (%). Pearson correlation coefficient and Spearman rank correlation coefficient were calculated for various parameters. Cutoff levels for different cardiovascular biomarkers were determined. For a particular cutoff level sensitivity, specificity of biomarkers was calculated and area under the receiver operating characteristic curve (AUROC) was plotted for biomarkers in relation to CIMT. Comparisons among three CKD stages were assessed by univariate analysis of variance (ANOVA). Chi-squared test was applied to make out the difference between the percentages of two sets of values. P value < 0.05 was considered as statistically significant.
4. Results
Table 1 presents the clinical characteristics for all patients in CKD stages.
| Variable | All Stages | Stage 3 | Stage 4 | Stage 5 | P Value |
|---|---|---|---|---|---|
| Number of patients | 120 | 40 | 40 | 40 | |
| Gender | Ns | ||||
| Male | 70 | 27 | 25 | 18 | |
| Female | 50 | 13 | 15 | 22 | |
| Age, y | 46.73 ± 14.18 | 44.68 ± 15.22 | 47.38 ± 14.39 | 48.05 ± 12.96 | Ns |
| Duration of CKD, y | 2.98 ± 1.47 | 2.25 ± 1.19 | 3.23 ± 1.49 | 3.48 ± 1.46 | < 0.001 |
| eGFR, mL/min/1.73 m2 | 24.16 ± 12.17 | 39.21 ± 16.48 | 21.30 ± 3.75 | 11.98 ± 1.73 | < 0.0001 |
| Serum creatinine, mg/dL | 3.27 ± 1.34 | 1.85 ± 0.24 | 3.12 ± 0.54 | 4.83 ± 0.73 | < 0.0001 |
| SBP, mmHg | 135.60 ± 11.80 | 135.38 ± 12.24 | 135.05 ± 11.52 | 136.38 ± 11.90 | Ns |
| DBP, mmHg | 83.16 ± 6.87 | 83.55 ± 6.99 | 82.35 ± 7.30 | 83.58 ± 6.39 | Ns |
| On any antihypertensive, % | 80.83 | 72.5 | 80 | 90 | Ns |
| H/O CAD, % | 39.16 | 32.5 | 37.5 | 47.5 | Ns |
| H/O smoking, % | 31 | 35 | 30 | 27.5 | Ns |
| Diabetes mellitus, % | 40 | 37.5 | 37.5 | 45 | Ns |
| BSA, m2 | 1.61 ± 0.11 | 1.60 ± 0.11 | 1.62 ± 0.12 | 1.62 ± 0.11 | Ns |
| CIMT, mm | 0.78 ± 0.15 | 0.77 ± 0.15 | 0.77 ± 0.16 | 0.79 ± 0.16 | Ns |
| BMI, kg/m2 | 22.68 ± 2.48 | 22.20 ± 2.01 | 22.99 ± 2.85 | 22.84 ± 2.50 | Ns |
| Hemoglobin, g/dL | 10.80 ± 1.44 | 11.22 ± 1.38 | 10.83 ± 1.34 | 10.34 ± 1.51 | 0.024 |
| Intact PTH, pg/mL | 154.03 ± 82.78 | 87.85 ± 25.27 | 149.28 ± 51.74 | 224.98 ± 88.97 | < 0.0001 |
| Serum cholesterol, mg/dL | 167.10 ± 43.89 | 174.80 ± 43.60 | 155.90 ± 41.50 | 170.60 ± 45.31 | Ns |
| Patients on statins, % | 48.33 | 42.5 | 50 | 52.5 | Ns |
| Plasma NT-proBNP, pg/mL | 585.68 ± 514.84 | 350.30 ± 277.34 | 613.35 ± 561.51 | 793.40 ± 560.98 | < 0.0001 |
| Serum cTnT > 0.01 ng/mL, % | 40 | 22.5 | 37.5 | 60 | 0.002 |
| Serum hs-CRP, mg/L | 5.96 ± 2.52 | 5.91 ± 2.68 | 5.94 ± 2.62 | 5.99 ± 2.31 | Ns |
| Spot urine ACR, mg/g of creatinine | 719.37 ± 411.36 | 394.58 ± 261.43 | 740.43 ± 366.39 | 1023.10 ± 332.39 | < 0.0001 |
a Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; H/O, history of; CAD, coronary artery disease; BSA, body surface area; CIMT, carotid intima media thickness; BMI, body mass index; PTH, parathormone; Ns, not significant; ACR, albumin creatinine ratio.
b Data are presented as Mean ± SD.
4.1. CKD Stage and Cardiac Biomarkers Concentrations
There was a significant trend toward increasing plasma NT-proBNP (P < 0.0001), serum cTnT (P = 0.002), and spot urine albumin creatinine ratio (P < 0.0001) concentrations with declining estimated GFR (eGFR). Median plasma NT-proBNP, median serum cTnT, and median spot urine ACR concentrations increased across CKD stages in the order of CKD stage 3, stage 4, and stage 5 (Table 1). Median serum hs-CRP concentrations did not differ significantly between patients with CKD stages 3, 4, and 5.
4.2. Overall Correlations of Cardiovascular Biomarkers and Other Variables With Each Other and CIMT
Regarding patients with all CKD stages, age, duration of CKD, systolic blood pressure, serum i-PTH, serum hs-CRP, plasma NT-proBNP, serum cTnT, and spot urine ACR correlated positively and significantly with carotid intima media thickness. However, hemoglobin and eGFR correlated negatively with CIMT (Table 2), and eGFR did not correlate significantly with the CIMT. In our study, plasma NT-proBNP, serum cTnT, and serum hs-CRP did not correlate significantly with age, sex, lipid profile, serum albumin, serum calcium, serum alkaline phosphatase, serum PO4, serum i-PTH, and body surface area.
| Variable | CIMT, mm | |
|---|---|---|
| r a | P Value | |
| Age, y b | 0.400 | 0.0001 |
| Duration of CKD, y c | 0.200 | 0.029 |
| eGFR, mL/min/1.73 m2 BSA b | -0.064 | Ns |
| Systolic BP, mmHg | 0.291 | 0.001 |
| Diastolic BP, mmHg | 0.112 | 0.224 |
| Hemoglobin, g/dL | -0.490 | 0.0001 |
| Blood urea, mg/dL | Ns | |
| Serum creatinine, mg/dL b | Ns | |
| Serum intact-PTH, pg/mL b | 0.189 | 0.039 |
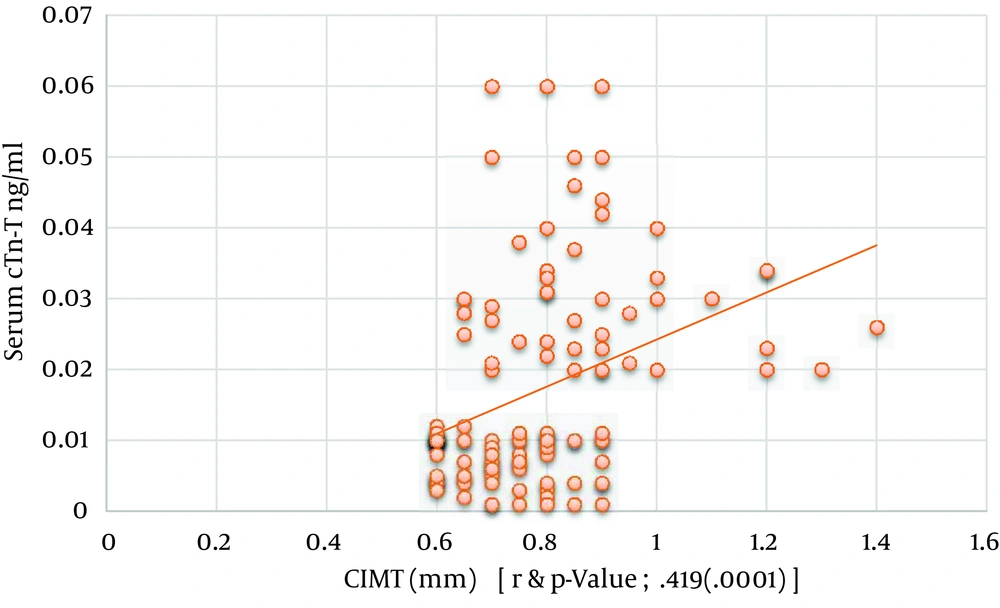
| Serum cTnT, > 0.01 ng/mL c | 0.419 | 0.0001 |
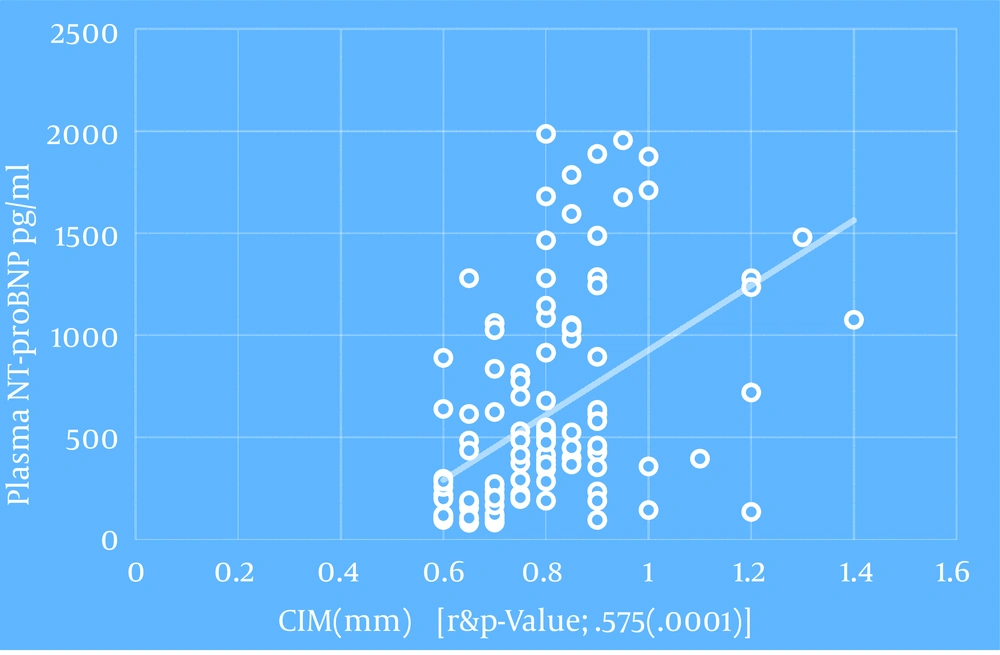
| Plasma NT-proBNP, pg/mL c | 0.575 | 0.0001 |
| Serum hs-CRP, mg/L c | 0.246 | 0.007 |
| Spot urine ACR, mg /g of creatinine c | 0.322 | 0.0001 |
a Correlation coefficient.
b Pearson correlation coefficient.
c Spearman Rho correlation coefficient.
4.3. CKD Stage Wise Correlations of Cardiovascular Biomarkers With CIMT
Hemoglobin, plasma NT-proBNP, serum cTnT, serum hs-CRP, and spot urine ACR correlated significantly with carotid intima media thickness in all three CKD stages (Table 3).
| Variable | CKD Stage 3 CIMT, mm | CKD Stage 4 CIMT, mm | CKD Stage 5 CIMT, mm | |||
|---|---|---|---|---|---|---|
| r a | P Value | r a | P Value | r a | P Value | |
| Hb, g/dL% b | -0.479 | 0.002 | -0.634 | 0.0001 | -0.390 | 0.013 |
| Serum cTnT > 0.01 ng/mL | 0.593 | 0.0001 b | 0.589 | 0.0001 c | 0.590 | 0.0001 c |
| Plasma NT-pro-BNP, pg/mL c | 0.533 | 0.0001 | 0.694 | 0.0001 | 0.509 | 0.0001 |
| Serum hs-CRP, mg/L c | 0.331 | 0.037 | 0.400 | 0.010 | 0.401 | 0.010 |
| Urine ACR, mg/g of creatinine c | 0.528 | 0.0001 | 0.505 | 0.0001 | 0.546 | 0.0001 |
a Correlation.
b Pearson correlation coefficient.
c Spearman Rho correlation coefficient.
4.4. Correlation Matrix Table for Cardiovascular Biomarkers
Biomarkers were interrelated, also they were related with CIMT as shown in Table 4.
a Spearman Rho correlation coefficient.
4.5. Sensitivity, Specificity and Area Under the Receiver Operating Characteristic Curve (AUROC) for Biomarkers in Relation to CIMT
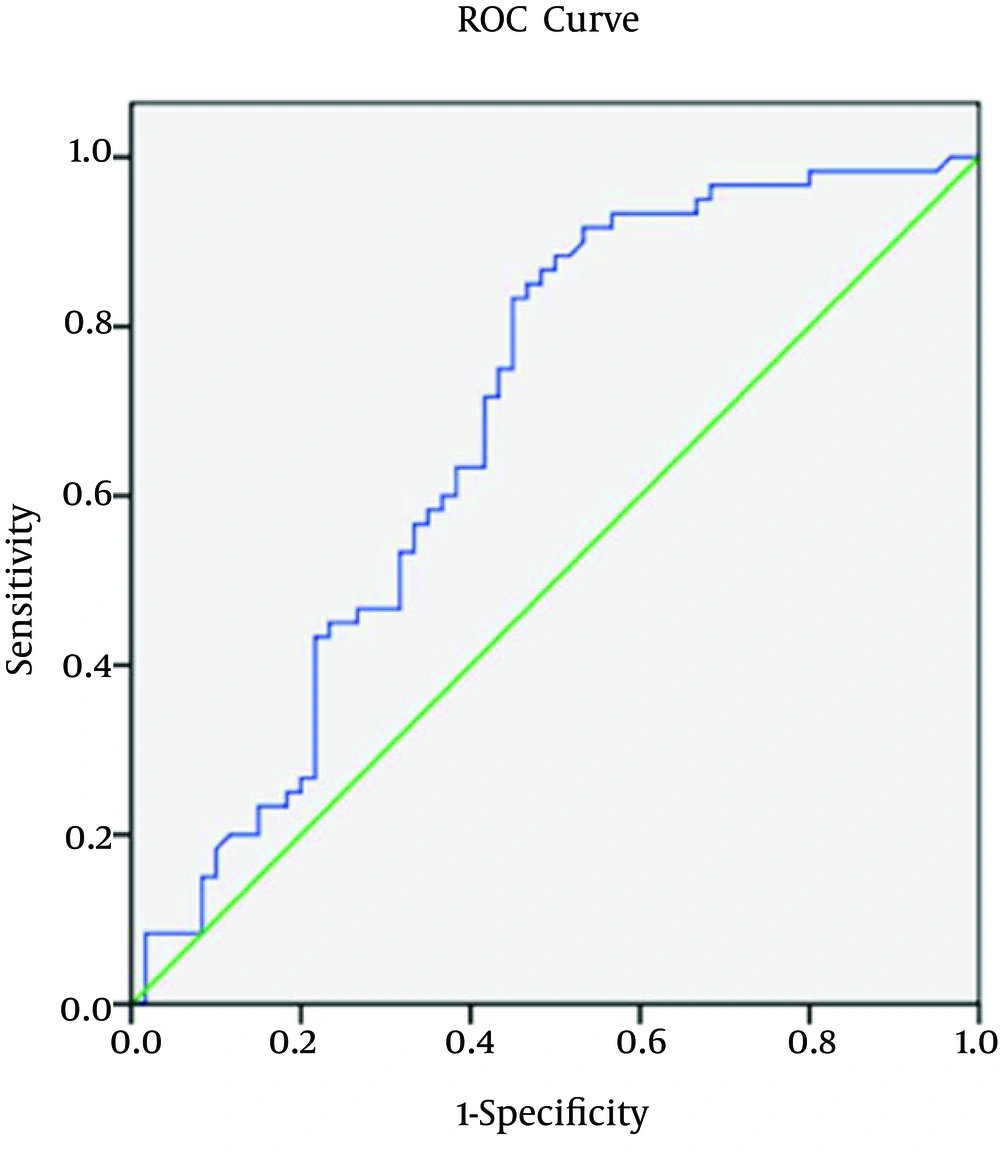
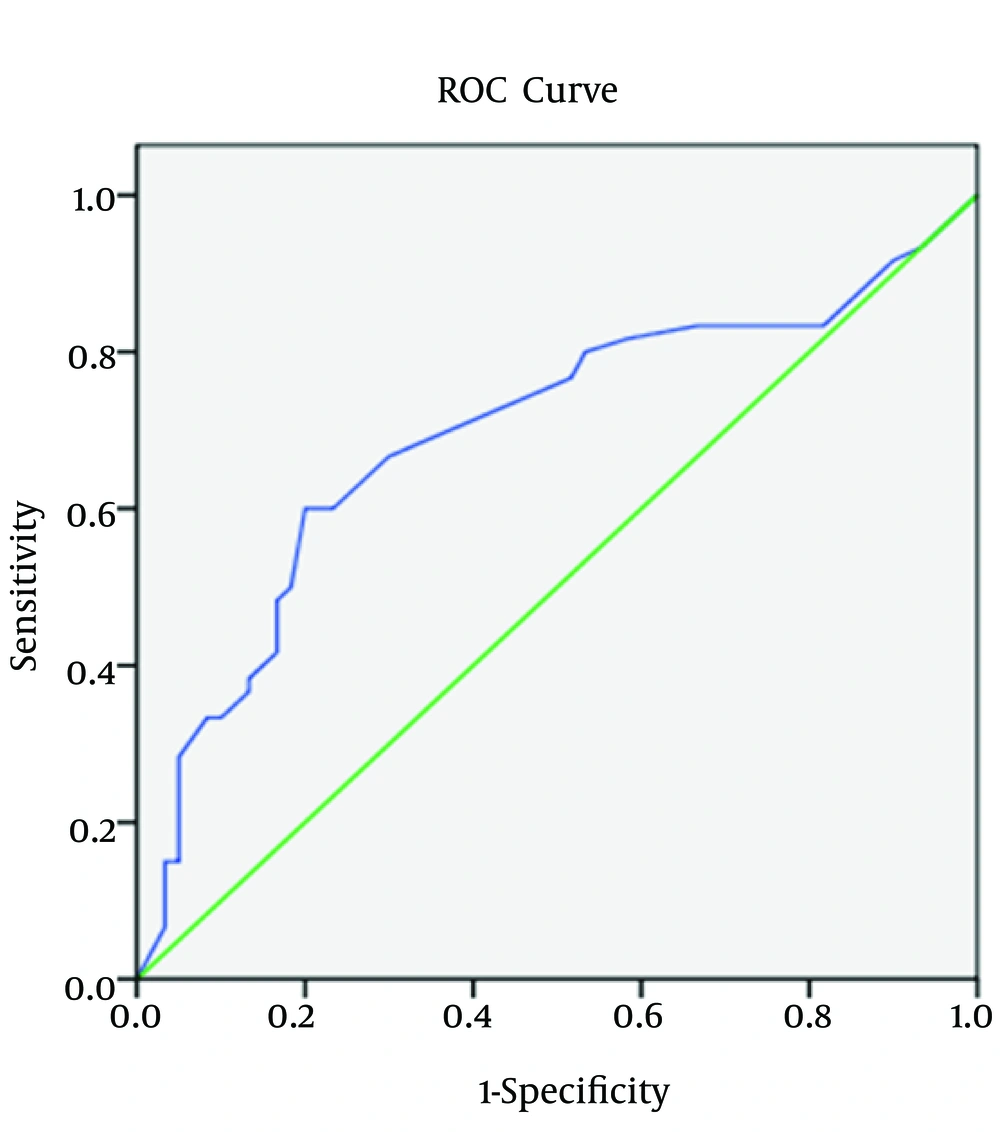
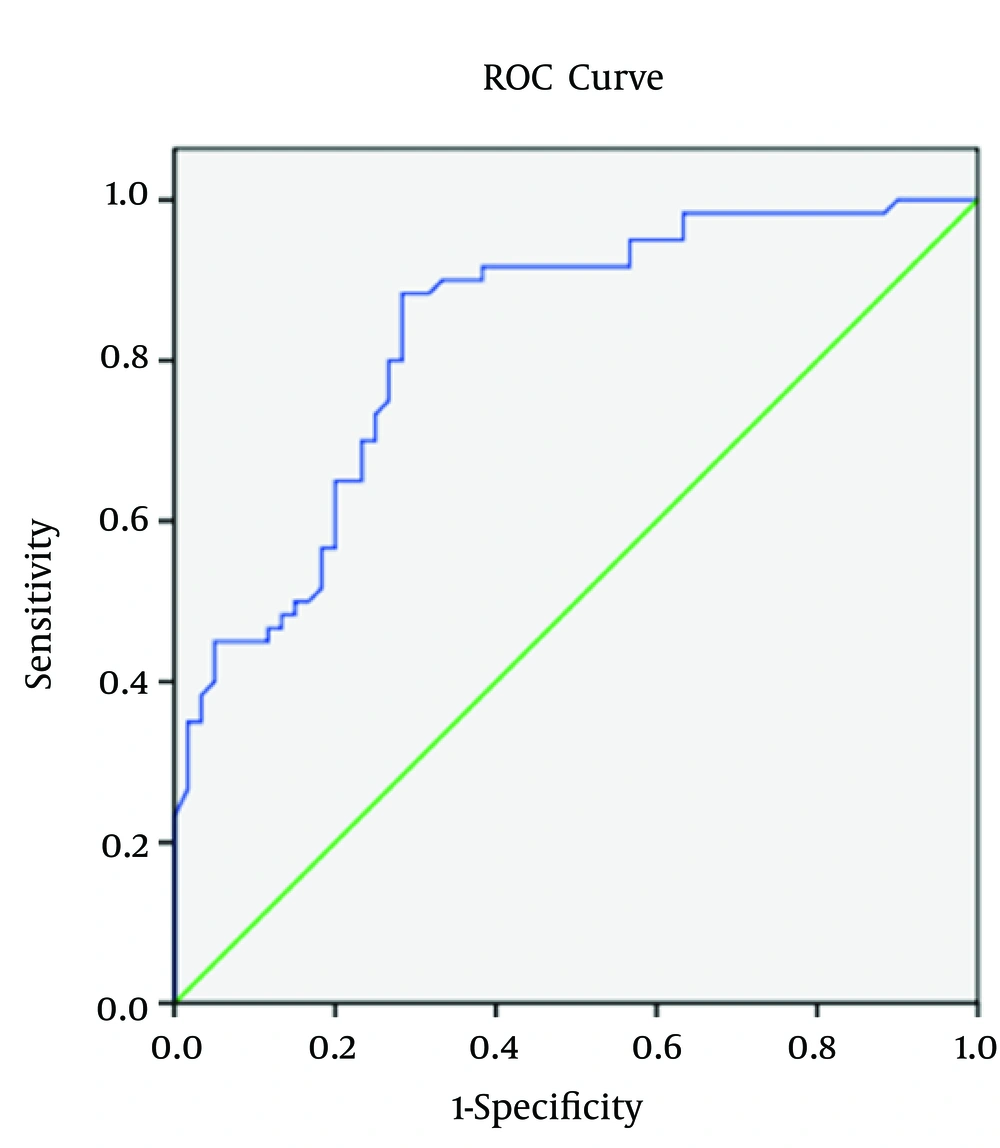
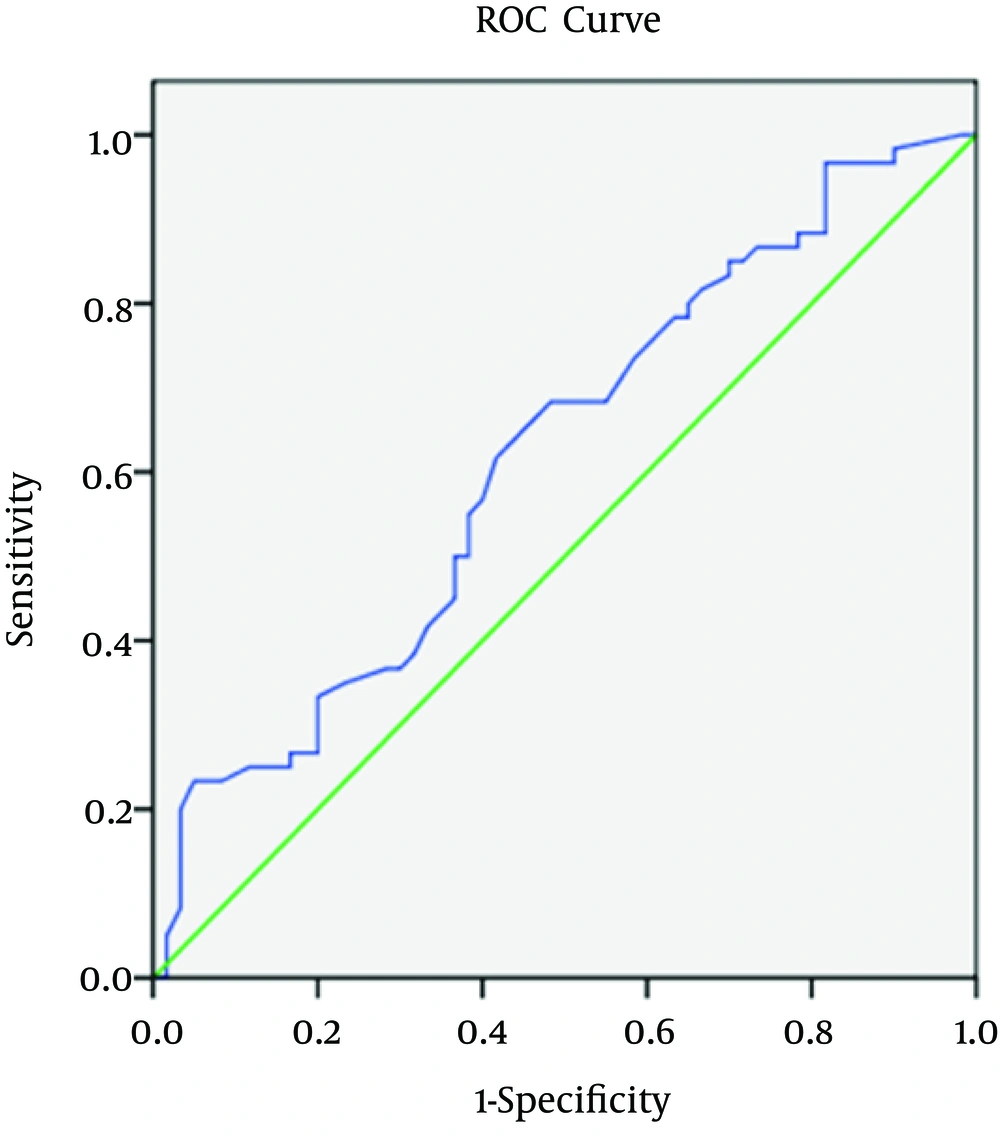
Regarding spot urine albumin creatinine ratio, at cutoff level of 678 mg/g of creatinine, the sensitivity, specificity, and AUROC (95% CI) were 71%, 58.3%, and 0.685 (0.58 to 0.82), respectively in relation to CIMT (Figure 1-3). 1-3). With regard to serum cTnT, at cutoff level of 0.010 ng/mL, the sensitivity, specificity, and AUROC (95% CI) were 67%, 70%, and 0.695 (0.53 to 0.78), respectively in relation to CIMT (Figure 4). For plasma NT-proBNP, at cutoff level of 321 pg/mL, the sensitivity, specificity, and AUROC (95% CI) were 88%, 71.7%, and 0.831 (0.77 to 0.95), respectively in relation to CIMT (Figure 5). Finally, regarding serum hs-CRP, at cutoff level of 5.7 mg/L, the sensitivity, specificity, and AUROC (95% CI) were 65%, 55%, and 0.618 (0.51 to 0.76), respectively in relation to CIMT (Figure 6).
5. Discussion
Recently, kidney disease has drawn attention as a cardiac risk factor with the importance similar to diabetes and history of prior cardiac disease (2). However, estimation of cardiac risk in asymptomatic predialysis CKD population remains challenging due to the high prevalence of traditional cardiac risk factors. If we could detect significant cardiac disease at an early stage, it would facilitate more aggressive and focused treatment of those at increased risk. Since the atherosclerotic changes in the carotid artery mirror general atherosclerosis (21), ultrasound measurements of the intima media thickness (IMT) in the carotid arteries were used as an indicator of coronary atherosclerosis (21). In fact, a close relationship exists between carotid artery wall morphology and CVD (21). B mode Ultrasound measurements of the intima media thickness (IMT) in the carotid arteries predicts strongly for cardiovascular events in the general population and dialysis patients (22). However, it is unclear whether carotid IMT is useful for the prediction of cardiovascular events in predialysis patients with CKD.
The present study enlightens the association of elevated cardiovascular biomarkers with the documented pathological states (increased carotid intima media thickness) in asymptomatic predialysis CKD patients. Among cardiovascular biomarkers, plasma NT-proBNP had maximum correlation (Spearman Rho correlation coefficient; r = 0.575 and P < 0.0001) with the carotid intima media thickness followed by serum cTnT (Spearman Rho correlation coefficient; r = 0.419 and P < 0.0001), spot urine albumin creatinine ratio (Spearman Rho correlation coefficient; r = 0.322 and P < 0.0001), and serum hs-CRP (Spearman Rho correlation coefficient; r = 0.246 and P = 0.007).
Previously, it was thought that role of plasma NT-proBNP has been restricted to diagnose heart failure in patients with symptoms of dyspnea (23). However, there is evidence that NT-proBNP level increases in response to isolated ischemia and myocardial injury even in the absence of heart failure (24). In our study, NT-proBNP levels were elevated in most patients with known coronary artery disease, which was consistent with previous work of DeFilippi et al. (25). NT-proBNP levels appear to increase to a greater degree in patients with CKD with underlying coronary artery disease (25). Patients with lower eGFR had high plasma NT-proBNP levels, which were consistent with a study done by Desai et al. (26).
Levels of cardiac troponin are frequently elevated in the absence of acute coronary syndrome among patients with varying degrees of kidney disease (27). For predialysis CKD patients, however, data are limited and conflicting. In our study, the prevalence of increased serum cTnT in predialysis CKD patients were 22.5%, 37.5%, and 60% in CKD stage 3, stage 4, and stage 5, respectively, which was consistent with the studied done by Abbas et al. and Landry et al. on CRIB (27, 28). Serum cTnT was more likely to be increased in patients with diabetes (33 of 48), which was consistent with the study done by Abbas et al. (27). Serum cTnT was more likely to be increased in patients with history of coronary artery disease (38 of 47). Serum cTnT correlated positively and significantly with the carotid intima media thickness (r = 0.419 and P value = 0.0001), which was consistent with the study done by Caliskan et al. (29).
There are multiple associations between CRP levels and CVD outcomes in patients with CKD. It is associated with increased mortality across the full spectrum of CKD (30). Also, an hs-CRP concentration > 3 mg/L is a strong risk factor for a coronary event (10). Serum hs-CRP did not correlate with eGFR in our study, which was consistent with the study of Christopher et al. (25). Serum hs-CRP was correlated positively and significantly with CIMT (r = 0.246 and P value = 0.007), which was consistent with the study of Szeto et al. (31). Serum hs-CRP correlated positively and significantly with spot urine albumin creatinine ratio (r = 0.192 and P value = 0.035).
In general, albumin to creatinine ratio has been shown to have slightly better diagnostic accuracy than urine albumin concentration alone for detection of albuminuria in many populations (32). In addition to predicting CKD progression, proteinuria is a well-established risk marker for cardiovascular disease (33). In our study, spot urine albumin creatinine ratio was > 30 mg/g of creatinine in most predialysis CKD patients, which was consistent with the study done by Landry et al. (28). Spot urine albumin creatinine ratio was correlated positively and significantly with the carotid intima media thickness (r = 0.332 and P = 0.0001). Among cardiovascular biomarkers, plasma NT-proBNP had maximum strength of positive correlation (Spearman Rho correlation coefficient; r = 0.610) with spot urine ACR (P = 0.0001). Spot urine albumin creatinine ratio was correlated positively and significantly with serum cTnT (r = 0.484 and P value = 0.0001), and with plasma NT-proBNP (r = 0.610 and P = 0.0001), which was consistent with the study done by Desai et al. (26). Out of 120 predialysis CKD patients, 41 patients (34.16%) had spot urine albumin creatinine ratio greater than 1 g/g of creatinine. Out of 41 patients with urine ACR greater than 1g/g of creatinine, 28 patients (68%) had increased serum cTnT and 40 patients (97.6%) had increased plasma NT-proBNP level. Spot urine albumin creatinine ratio was correlated negatively and significantly with the hemoglobin (r = -0.507 and P value = .0001) and with eGFR (r = -0.641 and P = 0.0001), which was consistent with the study done by Desai et al. (26).
Mean carotid intima media thickness (CIMT) was 0.78 ± 0.15 mm in our predialysis CKD patients, which was consistent with the study done by Szeto et al. In predialysis Chinese CKD patients, CIMT was 0.80 ± 0.19 mm (31). Modi et al. showed that CIMT higher than 0.75 mm was a strong predictor of CAD in Indian patients with end stage renal disease and can be used as a noninvasive screening test for pretransplant cardiac evaluation with high sensitivity, specificity and positive predictive value (20). Prevalence of CIMT (> 0.75 mm) was 50% (60/120) in our predialysis CKD patients. Prevalence of increased CIMT was 47.5%, 50%, and 52.5% in CKD stage 3, stage 4 and stage 5, respectively. Prevalence of increased CIMT was not statistically significant among three CKD stages. Carotid intima media thickness did not correlate significantly with eGFR, which was consistent with Szeto et al. study in predialysis Chinese CKD patients (31). Out of 48 patients who were diabetic, 33 patients (68.8%) had increased CIMT (> 0.75 mm), which was statistically significant (P ≤ 0.0001) and consistent with the study done by Modi et al. (20). The Szeto et al. study in predialysis Chinese CKD patients also showed that carotid IMT was significantly higher in patients with diabetes (31). Out of 47 patients with the history of coronary artery disease, 37 patients (78.7%) had increased CIMT, which was statistically significant (P ≤ 0.0001), and was consistent with the study done by Modi et al. in end stage renal disease Indian patients (20). Carotid intima media thickness correlated negatively and significantly with the hemoglobin concentration (P = 0.0001). Carotid intima media thickness correlated positively and significantly with the age, duration of CKD, and systolic blood pressure. In our study, carotid intima media thickness did not correlate significantly with sex, history of smoking, lipid profile, serum albumin, serum calcium, serum alkaline phosphatase, serum PO4, serum i-PTH, and body surface area. Previous studies did not find a relation between carotid IMT and blood pressure or serum calcium-phosphate product in long-term hemodialysis patients (17, 34). The absolute values of CIMT in our patients were smaller than those reported by Kato et al., Benedetto et al. and Nishizawa et al. (17, 18, 35). It is important to note that these studies examined patients with long-term dialysis, whereas our study had enrolled only predialysis CKD patients. Taken together, our results indicate that increase in CIMT occurs early in the course of CKD (stage 3 in our study). Although it is commonly believed that atherosclerosis is less severe in Asian patients, previous studies in Japanese patients did not show a lower IMT compared to white population (15, 18, 34, 35). In this study, carotid intima media thickness also correlates with diabetic status, duration of CKD, and history of coronary artery disease, which are not unexpected findings.
Our present work showed that declining kidney function causes elevation of cardiovascular biomarkers early (CKD stage 3 in our study) and there is a significant association of nontraditional cardiovascular biomarkers with the carotid intima media thickness. These biomarkers might be used to estimate cardiovascular risk in a predialysis CKD population with expected high cardiac morbidity and mortality.
Patients at CKD stages 1 and 2 were not included in the study. Relatively a small number of predialysis CKD patients were enrolled in the study because of the budget limitations and study design. Role of other novel cardiovascular biomarkers like FGF-23 (Fibroblast growth factor-23) and risk factors of premature atherosclerosis, such as serum homocysteine and lipoprotein were not studied because of budget limitations too. Serial monitoring of novel cardiovascular biomarkers with long follow up will better stratify and predict the cardiovascular risk in predialysis CKD patients. Most patients were under regular treatment on diuretics, antihypertensive drugs, statins, vitamin D, phosphate binders, and antiproteinuric drugs. Thus, medication might have confounded some results. Larger prospective multicenter trials should be conducted in predialysis patients to confirm the results of the present study.