1. Background
Kidney transplantation has long been recognized as the best available therapy for end-stage kidney disease. While operative technique has been consistently refined over time and is now highly standardized, there is ongoing research on details of the procedure, possibly leading to a further reduction of postoperative complications and improvement of the long-term outcome.
Urologic complications after renal transplantation include urinary obstruction of the transplant ureter due to a variety of causes including stenosis or kinking of the transplant ureter, stenosis or insufficiency of the ureterovesical anastomosis, obstruction by blood clots from macrohematuria, and urinary tract infections (UTIs) (1).
Routine intraoperative ureteral stenting has been shown to reduce postoperative complications from stenosis or kinking of the transplant ureter and necrosis and insufficiency of the ureterovesical anastomosis (2-5). Routine urinary stenting has also been shown to be cost effective, at least if the stent is removed on day 30 after transplantation (4, 6).
However, there is an ongoing debate as to whether routine placement of a urinary stent could lead to an increase in a different set of postoperative complications including UTIs, vesicoureteral reflux, prolonged duration of postoperative macrohematuria, pressure necrosis of the ureter, obstruction of the stent leading to hydronephrosis of the donor organ, dislocation of the stent, and a troublesome foreign body sensation reported by some patients (7, 8).
Internal, double-J ureteric stents do not have an open leg outside of the body and could be expected to provide protection of urinary flow and could be expected to ensure urinary flow while providing protection to the ureterovesicular anastomosis, with a reduced rate of infectious complications when compared to externalized uretero-vesico-cutaneous stents. Additionally, due to their internal placement, double-J catheters can be left in situ for longer periods of time, possibly providing prolonged protection from postoperative complications (9).
Percutaneous stents had been consistently used at our institution for the given advantages, including the ease of estimating the grafts excretory function, easy radiological examination of bladder and ureteroneocystostomy with contrast agent administered via the external opening, and simplicity of stent removal.
In light of mounting evidence for significantly reduced postoperative complications associated with the use of indwelling urinary stents in adult (9) and pediatric (10) renal transplant patients, our strategy concerning the use of ureteral stents changed to using double-J-style catheters for standard urinary stenting in kidney transplantation.
2. Objectives
This study aimed to compare the rate of postoperative complications occurring with both types of urinary stents to determine whether the use of internal JJ-type stents improves the outcome of adult renal transplantation when compared with external (uretero-vesico-cutaneous) stents.
3. Patients and Methods
This study was conducted as a retrospective analysis of all consecutive renal transplantations performed at our department within one year. From a total of 79 patients underwent renal transplantation during the study period, three patients who received multivisceral transplantations were excluded.
Details about the remaining 76 transplantations were retrieved from the renal transplant database, as well as by manual chart review of clinical, biochemical and radiological records. There were 61 primary transplants, 11 second transplants, 3 third transplants and one 4th transplant in the series. Thirteen donor organs were from living-related donors and 63 from deceased organ donors.
A team of three experienced surgeons performed all transplantations using a single standardized operating technique. Donor organs were transplanted into the iliac fossa of the recipient using an extra peritoneal approach with arterial and venous anastomoses to the iliac vessels. Urinary continuity was established using a modified Lich-Gregoire antireflux ureteroneocystostomy. All patients received intraoperative stenting of the transplant ureter, either by external percutaneous transcystic stenting (group 1, n = 43), or by internal (Double-J) stenting (group 2, n = 33). Additionally, an indwelling transurethral bladder catheter was placed in all patients for an average of 6 days postoperatively.
Externally draining uretero-vesico-cutaneous stents were left in situ for a median of 10 days. Patients in group 2 had a 4.7 F, 8.22 cm double-J in-dwelling uretero-vesical stent (Cook Urological, Spencer, IN, USA) inserted for a median of 51 days.
Baseline immunosuppression consisted of calcineurin inhibitors (CsA or tacrolimus), corticosteroids and Mycophenolate Mofetil (MMF) and was given to all recipients. Additionally, induction therapy with anti-CD25 monoclonal antibodies (basiliximab 20 mg) was given in 22 cases in group 1 and 21 cases in group 2 on day 0 and day 4.
Baseline serum creatinine levels were recorded preoperatively, and on a daily basis after transplantation. Color-coded duplex sonography was performed daily for the first postoperative week and as needed afterwards for the assessment of organ perfusion and vascular resistance, as well as for the exclusion of postoperative complications including hydroureter and/or hydronephrosis, hematoma and anastomotic insufficiency.
Endpoints of this study were postoperative complications including UTIs, ureteric stenosis or obstruction, anastomotic leakage and macrohematuria. In addition, the length of hospital stay was evaluated and compared between the two groups.
Diagnosis of urinary tract infection was based on was based on criteria specified by the centers for disease control and prevention (CDC) (11) and defined as a microbial count of more than 105 microorganisms/µL in conjunction with at least one of the following symptoms as experienced by the patient with no other recognized cause: fever (> 38°C), urgency, frequency, dysuria, or suprapubic tenderness. Ureteric stenosis was defined as a rise in serum creatinine by more than 20%, ultrasonographic evidence for hydronephrosis and verification of the stenotic anastomosis by retrograde pyelography. If no relevant stenosis was discovered or there was direct evidence of obstruction by another cause (urolithiasis, thrombotic material), the complication was defined as ureteric obstruction. Anastomotic leakage was defined as any amount of contrast agent outside the transplant ureter or bladder detected by retrograde pyelography, routinely performed on postoperative day 8. Secondary macrohematuria was defined as any macrohematuria newly arising after cessation of initial postoperative hematuria.
Statistical analysis was performed using the SPSS statistical software package, (SPSS Inc., Chicago IL, USA). Group means or medians were compared using the unpaired t-test, contingency tables were analyzed using Fisher’s exact test.
4. Results
In 76 kidney transplants 43 external (group1) and 33 double-J (group 2) stents were used. No significant demographic differences were observed between the two groups regarding patient age (median patient age of 50, range 18 - 73 for group 1 and a median age of 50, range 26 - 69 for group 2, P = 0.282), gender distribution (30.2 % females in group 1, 42.4 % in group 2, P = 0.998), BMI (median 25.1 vs. 24.9 in group 1 and 2, respectively, P = 0.795), percentage of living-related donors (20.9 % and 12.1 % in groups 1 and 2, respectively.) and number of donors over the age of 65 taking part in the eurotransplant seniors program (ESP, 9.3 % in group 1, and 12.1 % in group 2). The duration of cold and warm ischemia in the donor organ did not differ significantly between the groups (Table 1).
| Characteristics | Group 1, External stent (n = 43) | Group 2, Double-J (n = 33) | P Value |
|---|---|---|---|
| Median age at transplantation, (range) | 50 (18-73) | 50 (26-69) | 0.282 |
| Gender | 0.998 | ||
| Male | 30 | 19 | |
| Female | 13 | 14 | |
| Median BMI, kg/m2 | 25.1 ± 3.8 | 24.9 ± 4.1 | 0.795 |
| Donor origin -living: | 0.370 | ||
| Related | 9 | 4 | |
| Cadaveric | 34 | 29 | |
| Old for old | 4 | 4 | 0.701 |
| Cold ischemia, min | 658 ± 335 | 650 ± 313 | 0.919 |
| Warm ischemia, min | 32 ± 7 | 30 ± 9 | 0.433 |
a Abbreviations: BMI, body mass index, ESP, European Senior Program.
Postoperative complication rates are shown in Table 2. There was no significant difference in the number of UTIs (n = 16 or 37.2 % in group 1, n = 17 or 51.5 % in group 2, P = 0.481), ureteric stenosis (n = 3 in group 1, n = 0 in group 2, P = 0.256) or ureteric necrosis (n = 1, 2.3 % in group 1, n = 0 in group 2, P = 1.00).
| Complication | Group 1, external stent (n = 43) | Group 2, double-J (n = 33) | P Value |
|---|---|---|---|
| Urinary tract infection | 16 (37.2) | 17 (51.5 %) | 0.481 |
| Ureteric stenosis | 3 (6.98) | 0 | 0.256 |
| Ureteral necrosis | 1 (2.33) | 0 | 1.000 |
| Urethral obstruction | 2 (4.6) | 0 | 0.504 |
| Anastomotic leakage | 6 (14) | 0 | 0.035 |
| Hematuria | 13 (30.23) | 17 (51.5) | 0.045 |
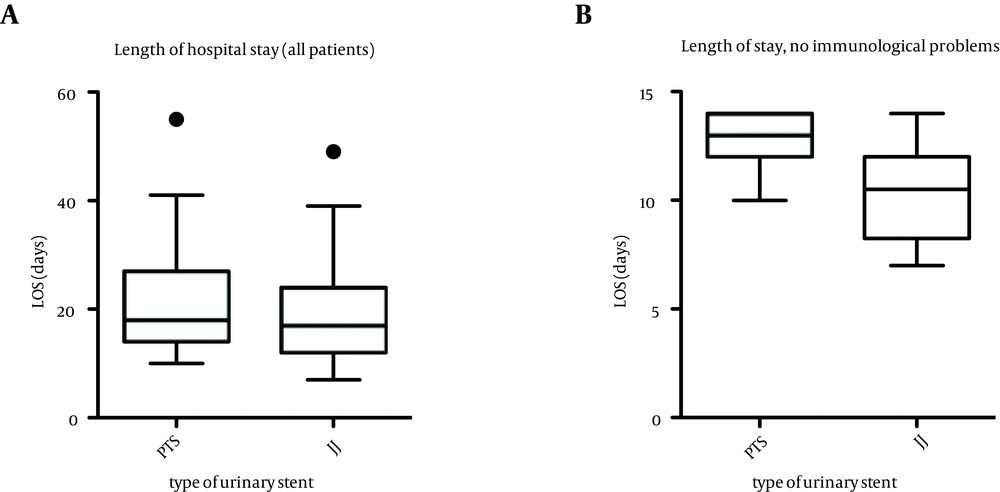
The overall mean length of hospital stay (Figure 1) was comparable in the two groups (19.3 days in group 1, 20.7 days in group 2, P = 0.533). However, the hospital stay of patients without immunological complications was significantly decreased using double-J stents (12.9 days in group 1, 10.8 days in group 2, P = 0.018). Ureteroneocystostomy leakage occurred in 6 out of the 43 patients in group 2 (13.9%), while no case of insufficient anastomosis was observed in group 1 (P = 0.035). There were significantly fewer occurrences of secondary macrohematuria in group 1 (3/33 patients) as compared to group 2 (13 of 43 patients, P = 0.045).
5. Discussion
The present study seems indicative of a lowered rate of postoperative urinary complications with the use of internally placed, double-J style ureterovesical stents when compared to percutaneous, uretero-vesico-cutaneous drainage.
Typical postoperative complications after kidney transplantation include obstruction, stenosis or kinking of the transplant ureter, insufficiency of the uretero-neo-vesicular anastomosis, as well as UTIs and postoperative macrohematuria, possibly leading to obstruction of urinary drainage and complications due to the heightened pressure on the transplant kidney as well as the anastomosis (8, 9, 12-14).
In the present study, we have not seen a significant difference in the rate of ureteric stenosis, ureteric obstruction or anastomotic insufficiency between the two groups. However, it should be noted that none of these complications occurred in internally stented patients at all. Complication rates of 7% for ureteric stenosis and 4.6 % for ureteric obstruction in the externally drained group are in line with prior studies (15), while an absence of each of these complications in the JJ group has previously been shown in large meta-analyses (3, 5).
Statistical relevance was shown concerning the rate of postoperative anastomotic insufficiency (14% of externally drained patients vs. 0% of internally drained patients). These results are in line with prior studies on adult and juvenile transplant recipients. In these, the rate of postoperative anastomotic insufficiency was shown to be ~ 10% in externally drained patients (10, 16-19), and nonexistent in patients with internal JJ-style drainage (3, 5). In our study, occurrence of all complications took place after removal of the external drainage; our hypothesis is that the longer duration of urinary stenting in the internally drained patients has a protective effect on the anastomosis. A recent study utilizing a 5-day external stenting protocol found this stenting period to be adequate for living donor transplant recipients only, while being insufficient for deceased donor transplantation (20).
We have found a high rate of secondary macrohematuria in patients with internal stents in the present study, while the rate of early postoperative hematuria did not differ between groups. No case of hematuria necessitated an operative revision, or led to a prolongation of hospital stay. In light of the protective effects of longer stent placement, hematuria may be an accepted disadvantage for the benefit of fewer complications.
The rate of postoperative UTIs did not differ significantly between patients with internal stenting and patients receiving external urinary drainage. In theory, externalized urinary stents could be accused of representing an additional entry point for microorganisms; therefore, heightening the risk of urinary tract infection, the additional placement of transurethral urinary catheters in recipients of JJ-style internal stents seems to suffice as an entryway, leading to the development of clinically significant UTIs (8).
In patients without immunological complications, length of hospital stay was significantly reduced in patients with JJ-stents as compared to externally drained patients, possibly reflecting the lower rate of urological complications in this group. In addition, JJ-style urinary stents do not have to be removed during the initial hospital stay, and later removal by cystoscopy can be performed in an outpatient setting.
In conclusion, the present study is indicative for an improved outcome with a lowered rate of postoperative urinary complications and shorter hospital stay of patients with internally placed, double-J uretero-vesical stents in kidney transplantation.