1. Background
A single instillation of mitomycin C (MMC) following transurethral resection of bladder tumour (TURBT) has been shown to reduce the recurrence of bladder cancer by up to 39% (1-3). Tumour recurrence following TURBT is thought to be due to cells from the resected tumour re-implanting into the urothelium following resection. Time from resection to recurrence is thought to be associated with tumour grade, lamina propria invasion and the presence of more than one tumour. The mechanism of action of intravesical chemotherapy involves destruction of circulating tumour cells by inhibiting DNA synthesis and ablation of the bladder cancer resection sites (4).
Sylvester et al. completed an initial meta-analysis in 2004 which showed an 11.7% reduction in recurrence and reduction of 39% in the odds of recurrence with PSDIVC (1). Mitomycin C, epirubicin, thiotepa, pirarubici and doxorubicin were all shown to have a beneficial effect (1).Two meta-analyses published in 2013 supported this evidence. Abern et al. found a 13% reduction in tumour recurrence using a single post-operative dose of IVC (2). Perlis et al. found a 12% reduction in early recurrence and 38% increase in the interval until recurrence but criticised 12 out of 13 studies for publication bias (3). These findings prompted endorsement by the European association of urology (EAU) and British association of urological surgeons to introduce guidelines suggesting that all patients with NMIBC undergoing TURBT should receive a post-operative instillation of intravesical chemotherapy within 24 hours (5, 6). Similarly the American Urological Association Guidelines recommend the use of PSDIVC for NMIBC perioperatively or postoperatively in an adjuvant fashion (7).
2. Objectives
We aim to audit the instillation of Mitomycin C at St Mary’s Hospital, London, against EAU guidelines, create an intervention bundle to overcome obstacles preventing administration and re-audit to show improvement in practice.
3. Patients and Methods
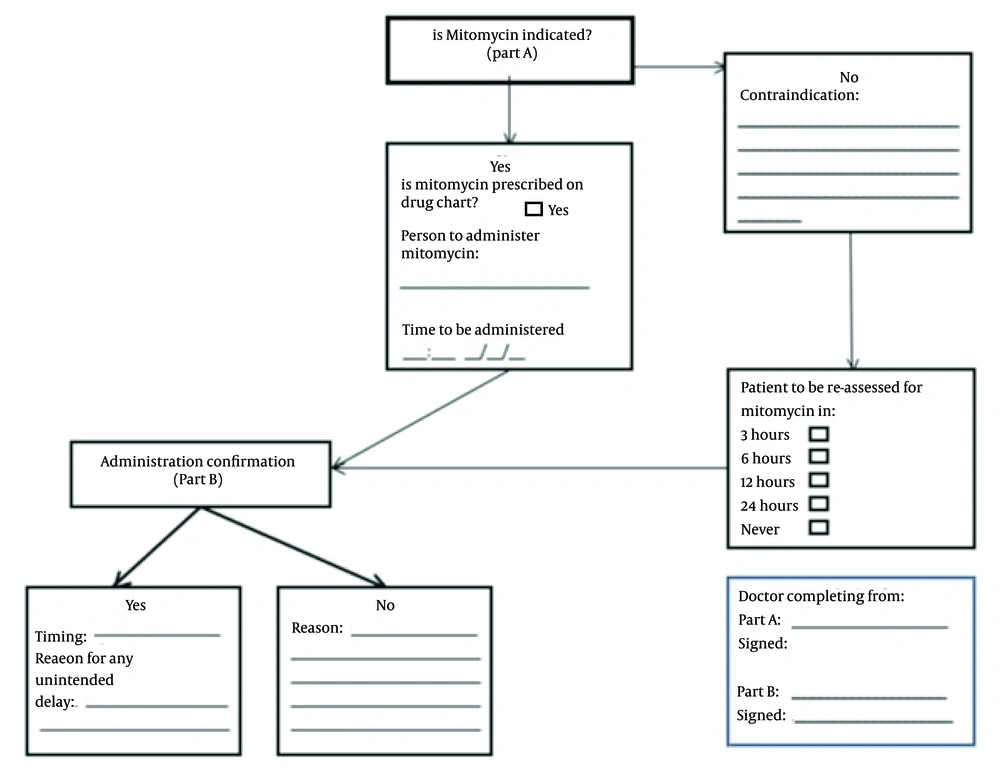
All patients that underwent TURBT over a 12-month period between March 2012 and February 2013 were allocated to group A and analysed to audit PSDIVC administration. Retrospective data was collected from operative notes, inpatient notes and post-operative medication charts. This data included indication for PSDIVC, whether instillation was given and documentation stating a contraindication if not given. Following discussion at a departmental meeting involving consultant urological surgeons, nurse practitioners and residents an intervention bundle was suggested. An intervention bundle including pre-operative delivery of MMC by the surgical resident to theatre suite, proforma placed pre-operatively in operation notes (Figure 1) and administration of MMC post-operatively by a surgical resident or nurse specialist was introduced, roles that were not previously clearly defined. Prospective re-audit data from group B was collected over a 6-month period between July 2013 and December 2013 in study two following intervention using the proforma.
4. Results
Sixty-four patients in group A underwent TURBT prior to the introduction of the intervention bundle (Table 1). Fifty-four patients had NMIBC which would have been eligible for PSDIVC. Fifteen (28% of NMIBC) were treated with intravesical mitomycin C post-operatively within 24 hours. Reasons why PSDIVC was not administered were documented in eight cases (Table 2). Mitomycin C was documented to be not available for three patients, contraindicated in four patients and prescribed but not given to one patient. Forty-one patients had no documented reason why mitomycin was not given. Twenty-three (36% of all patients) were either given PSDIVC or had a documented contraindication in group A.
| Group A | Group B | |
|---|---|---|
| Total number of TURBT | 64 | 31 |
| Non-muscle invasive disease (CIS, Ta, T1) | 54 | 24 |
| Muscle invasive disease (T2 and above) | 10 | 7 |
| Given PSDIVC within 24 hours | 15 | 12 |
| Given PSDIVC after 24 hours | 0 | 0 |
| Contraindication documented | 8 | 16 |
| No documented reason why not given | 41 | 3 |
Comparing Differences in Mitomycin C Administration Between Group A and Group B
| Reason Why PSDIVC Not Given | Group A | Group B |
|---|---|---|
| MMC not available | 3 | 0 |
| Palliative | 3 | 0 |
| Documented haematuria | 1 | 0 |
| Written in post-op notes but not given | 1 | 0 |
| Previously given adjuvant chemotherapy | 0 | 9 |
| Muscle invasive bladder cancer | 0 | 7 |
| No documented reason why PSDIVC not given | 41 | 3 |
| Total PSDIVC not given | 49 | 19 |
Documented Reasons Why PSDIVC Not Given
Thirty-one patients in group B underwent TURBT following induction of intervention bundle. Twenty-four patients had NMIBC. Twelve (50% of NMIBC) patients were given PSDIVC. Contraindications were documented for 16 patients; three had no reason documented as to why treatment was not administered. Twenty-eight (90% of all patients) in group B were either given PSDIVC or had a documented contraindication.
5. Discussion
This is the first audit performed in our department that has looked at MMC administration rates. The importance of early postoperative instillation has repeatedly been shown. Disappointingly administration rates in group A were low. As was the number of patients who had contraindications documented. The patients in group B had increased administration rates of PSDIVC and documentation levels.
Administration of PSDIVC was increased in group B when compared to group A. One of the possible reasons for this could include the increased availability of mitomycin C post-operatively – there were no patients in group B that did not receive mitomycin C compared to three in the first study due to lack of availability. Following the initial audit a pathway to ensure availability of mitomycin was introduced. As mitomycin is a controlled medication and used infrequently, pharmacy and nursing staff were reluctant to keep mitomycin in the theatre suite. Surgical residents ordered mitomycin from pharmacy pre-operatively and took responsibility for delivery to the theatre suite on the day of surgery. It was the responsibility of surgical residents or surgical nurse specialists to administer and document post-operatively, roles not previously determined. Ideally responsibility of pharmacy logistics would be taken over by pharmacy staff, allowing the surgical team to focus on other responsibilities. A further improvement to the intervention bundle could include pharmacy staff being made aware of the required medications pre-operatively and ensuring medications are delivered to theatre suite.
It is also worth noting that meta-analyses by Abern et al. (2) and Perlis et al. (3) were both published after group A, however earlier EAU guidelines published in 2011 recommend PSDIVC for non-muscle invasive bladder cancer (8). To increase awareness of EAU guidelines a departmental meeting was held where the results of group A were presented and urological surgeons and trainees reminded of adjuvant chemotherapy guidelines. Proformas placed pre-operatively in the operative notes acted as a reminder for the surgeon to consider mitomycin C on the day of the operation. In addition proformas acted as a method of improving documentation in group B.
Absolute contraindications for intravesical chemotherapy include post-operative bleeding, hypersensitivity, bladder perforation, myelosuppression, and thrombocytopenia (9). In both groups of the 27 documented reasons why mitomycin was not given only one was due to excessive bleeding. Nine patients were not given mitomycin due to recurrent disease which would be more suitable for Bacillus Calmette-Guerin (BCG) treatment. Seven patients were deemed not suitable for PSDIVC due to muscle invasive disease.
In both groups administration of PSDIVC was lower than expected. There has been some suggestion that PSDIVC in other centres has also been modest (3). A national study completed in the United States of 1010 patients with NMIBC found that only 16.9% were given a post-operative dose of intravesical chemotherapy (10). A national snapshot audit study in the United Kingdom showed more promising results. Gan et al. asked all urological surgeons in the UK to send details of one patient with newly diagnosis bladder cancer, 192 consultants replied, of which 61% of patients were given mitomycin C post-operatively (11).
Attitudes of practice towards IVC have also been slow to progress. A national survey of 269 urologists in the United States found that 61% never use post-operative IVC and only 8% use IVC frequently or always (10). Another survey of 104 urologists determined 5 key reasons why they were reluctant to give IVC following TURBT. These included (a) reluctance to give chemotherapy until histological confirmation (b) uncertainty of tumour invasiveness, (c) pharmacy logistics, (d) suspected bladder perforation and (e) toxicity (10). Lack of evidence was also stated as a possible reason why IVC was not given. Factors such as pharmacy logistics and lack of knowledge of recent evidence are obstacles that may be overcome to improve installation rates of post-operative MMC and improve adherence to EAU guidelines for NMIBC.
The obstacles we encountered in administration of adjuvant chemotherapy are likely to be shared in similar centres. Such intervention bundles could therefore be introduced at other urological centres to tackle obstacles such as awareness of indications for adjuvant chemotherapy, pathways for delivery of controlled medications and poor documentation.
Improvements were seen in rates of administration and documentation of contraindications to improve compliance with EAU guidelines. A pathway including improvement in pharmacy logistics and awareness of latest research may help to overcome barriers preventing the administration of post-operative adjuvant chemotherapy.