1. Background
Renal ischemia-reperfusion (IR) injury is major cause of acute kidney injury, a common clinical problem associated with an increasing prevalence and high mortality rate (1). The severity of the injury depends on the duration of ischemia and subsequent reperfusion phase. Reperfusion causes damage through generation of reactive oxygen species and inflammation rather than restoration of normal function (2). Therefore, the need for additional therapeutic modalities to prevent renal IR injury is quite urgent.
Erythropoietin is a glycoprotein hormone that was originally identified as the humoral factor, which control production of erythroid precursor cells. However, many evidence suggests that EPO has several functions independent of its effects on red blood cells production. Recently, studies in vitro and vivo have shown that EPO attenuates cell damage (3). The proper effects of the EPO-related changes are not fully clearly, even though it has anti-apoptotic, antioxidative and anti-inflammatory properties. Its pro-angiogenic potential seems to be related to EPO-mediated protective effect.
Ischemic preconditioning (IPC), defined as brief sublethal periods of ischemia followed by reperfusion before master ischemia, is a way to minimize subsequent events of IR injury (4, 5). Researches show that IPC regimen can protect the target organs or distant parts of organs and tissues (6, 7). This phenomenon has been demonstrated firstly in myocardium (8). Protective effects of IPC on IR injury have been frequently demonstrated in other organs such as skeletal muscle (9).
Antioxidants have been presented to be protective against IR interposed oxidative damage in different animal models (8). Lipid peroxidation refers to the oxidative deterioration of lipids and is one of the most important sources of oxidative stress. Several experimental studies have shown that lipid peroxidation occurred in renal IR insult (10). Therefore, ROS has been shown to contribute to the cellular damage induced by IR.
2. Objectives
The aim of this study was to compare the effects of recombinant human EPO and IPC on renal ischemia/reperfusion injury and their effects on the production of ROS.
3. Materials and Methods
3.1. Animals
Male Wistar albino rats, weighing 220 - 270 g, were used. Until the beginning of the research, rats were kept at room temperature (23°C - 25°C) and 40% - 60% relative humidity on a 12/12 hour light/dark cycle and were fed with standard pellet diet and water ad libitum. All procedures described below were performed under approval of the animal ethics committee of Tehran University of Medical Sciences.
3.2. Surgical Procedures
Rats were anaesthetized by an intraperitoneal administration mixture of ketamine (50 mg/kg) and xylazine (10 mg/kg). Rats were kept on heating pad to maintain their body temperature at 37°C. Systolic blood pressure and heart rate were recorded by a tail-cuff connected to a pulse transducer device (MLT125/R; AD Instruments, Castle Hill, NSW, Australia). The transducer was linked to a PowerLab/4SP data-acquisition system (Chart, version 5; AD Instruments). Rats with blood pressure below 60 mmHg were omitted from the study.
After satisfactory surgical anesthesia had been achieved, a midline laparotomy was done, in which the abdominal cavity was fully exposed. Bilateral renal pedicles were isolated carefully and clamped by non-crushing microvascular clamp to effect complete cessation of renal blood flow. After 50 minutes, the clamps were removed to allow return of blood flow to the kidneys, and then kidneys were undergone to reperfusion for 24 hours. The edges of the abdominal incision were joined to each other and covered by a piece of gauze soaked with warm isotonic saline (37°C) to prevent undue loss of body fluids. The abdomen was irrigated with warm isotonic saline, and then the abdominal wound was closed in two layers by continuous stitches.
The ischemic preconditioning (IPC) was performed by three cycles of alternating 3 minutes of bilateral renal pedicles ischemia and 3 minutes reperfusion. The occlusion was achieved by non-crushing microvascular clamp.
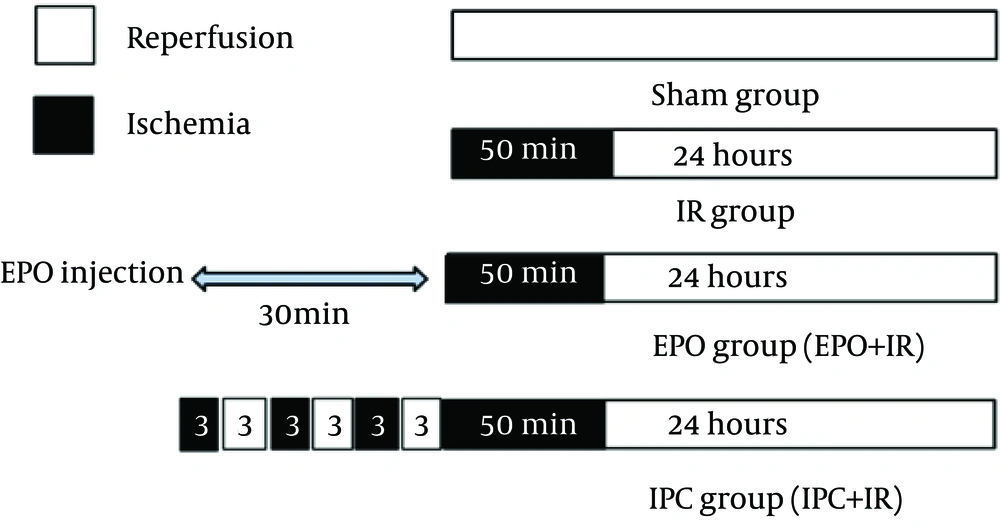
Rats were allocated randomly into four experimental groups (n = 6): Sham group, after laparotomy, rats were subjected to surgical manipulation without intervention; IR group, rats were subjected to bilaterally renal ischemia for 50 minutes followed by 24 hours reperfusion; IR + EPO, rats were subjected to IR (as in IR group), a single dose of EPO (5000 U/kg) was injected intraperitoneally thirty minutes before the onset of ischemia; IR + IPC, rats were subjected to ischemic preconditioning (IPC) regimen before the onset of ischemia (Figure 1).
At the end of the surgery, rats were kept in individual cages over a period of 24 hours. After 24 hours reperfusion, they were anaesthetized by mixture of ketamine (50 mg/kg) and xylazine (10 mg/kg) and blood samples were collected from the inferior vena cava. Rats were sacrificed and their kidneys were harvested before being washed and dissected in cold normal saline. Part of the left kidney was immediately snap-frozen in liquid nitrogen and stored at 70°C for renal oxidative stress status assay.
3.3. Renal Functional Assessments
Blood samples were centrifuged at 1,000 g for 15 minutes within 1 hour after collection. The plasma samples were stored in the -20°C freezer before they were analyzed. Plasma samples were analyzed for blood urea nitrogen (BUN) and plasma creatinine with commercial kits by an autoanalyzer.
3.4. Renal Oxidative Stress Assessments
3.4.1. Malondialdehyde Levels
Renal malondialdehyde (MDA) levels were determined by spectrophotometric methods (11). Kidney (50 mg) tissue samples were homogenized with trichloroacetic acid (TCA). Then, the homogenate was centrifuged at 3000 cycles for 15 minutes. Thiobarbituric acid (TBA) solution was added to the supernatant and boiled for 60 minutes. After samples were cooled down, the optical density of supernatant was measured at 532 nm.
3.4.2. Superoxide Dismutase Activity
To measure SOD activity, 50 mg of kidney tissue samples were homogenized in buffer phosphate. Homogenate was centrifuged at 3000 cycles for 15 minutes. A spectrophotometric method was used to determine renal SOD activity which was based on the inhibition of a superoxide-induced nicotinamide adenine dinucleotide oxidation. The optical density of supernatant was measured at 340 nm (12).
3.4.3. Reduced Glutathione Levels
Reduced glutathione (GSH) levels in kidney tissue were determined by spectrophotometery. Kidney tissue samples (50 mg) were homogenized in ethylenediaminetetraacetic acid (EDTA)-buffer phosphate and TCA solution. Homogenate was centrifuged at 3000 cycles for 15 minutes. The assay for GSH is based on the reaction of GSH with 5, 5’-dithiobis (2-nitrobenzoic acid), which produces the 2-nitro-5-thiobenzoic acid chromophore that can be monitored at 412 nm (13).
3.5. Statistical Analysis:
All values are expressed as mean ± SE. Continuous data between groups were compared by one-way ANOVA. ANOVA tests were followed by Tukey’s test. Differences with a value of P < 0.05 were considered statistically significant.
4. Results
4.1. Effect of Erythropoietin and Ischemic Preconditioning on Renal Function
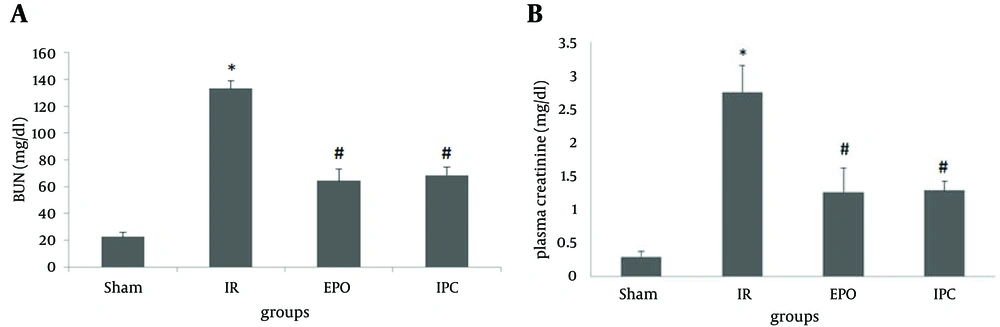
Bilateral renal ischemia (50 minutes) and 24 hours reperfusion resulted in a significant increase in plasma BUN compared with that in the sham group (22.50 ± 3.18 vs 133.33 ± 5.48 mg/dL respectively; P < 0.05) and an increase in plasma creatinine (0.29 ± 0.08 vs 2.75 ± 0.40 mg/dL, respectively; P < 0.05). Furthermore, EPO significantly decreased plasma BUN and creatinine (64.33 ± 8.75 mg/dL and 1.26 ± 0.36 mg/dL; respectively; both P < 0.05) compared to that in the IR group. Ischemic preconditioning also significantly decreased plasma BUN and creatinine (68.60 ± 5.87 mg/dL and 1.28 ± 0.14 mg/dL; respectively; both P < 0.05) compared to that in the IR group (Figure 2).
The sham group did not undergo any further interventions; the ischemia/reperfusion (IR) group was subjected to 50 minutes bilateral ischemia followed by 24 hours reperfusion. A single dose of EPO (5000 U/kg, i.p) was administered 30 minutes prior to ischemia. Ischemic preconditioning consisted of three cycles of 3 minutes intermittent IR of renal pedicles applied at the beginning of the 50 minutes period of renal ischemia, followed by 24 hours reperfusion. Data are the mean ± SEM (n = 6 to 9). *P < 0.05 compared to the sham group; #P < 0.05 compared to the IR group (one-way ANOVA followed by Tukey’s test).
4.2. Effects of Erythropoietin on Oxidative Stress in the Kidney
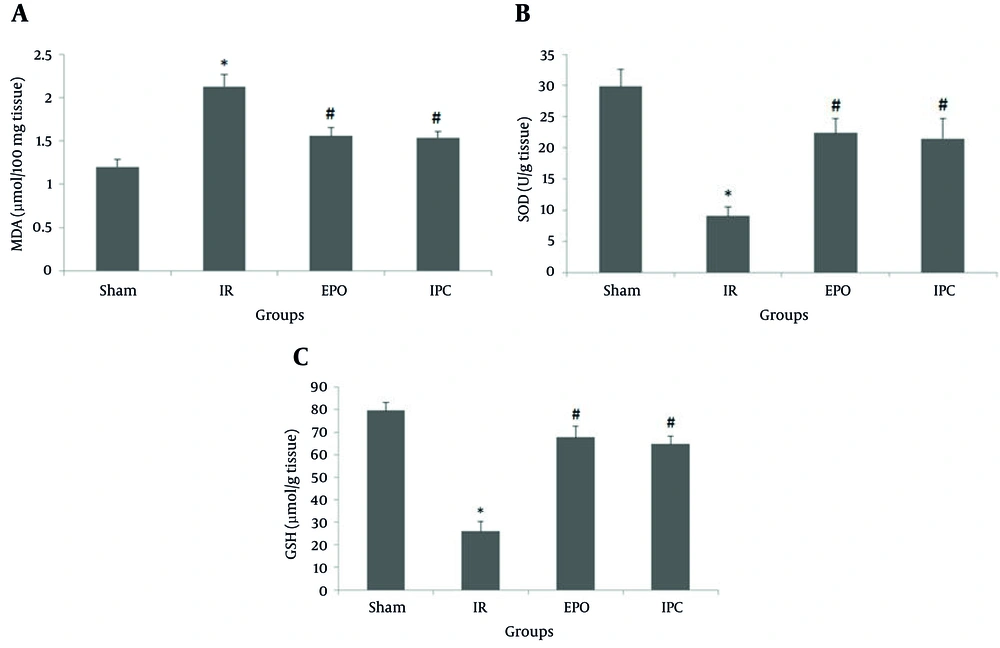
Bilateral renal ischemia (50 minutes) and 24 hours reperfusion resulted in significant increase in MDA content compared to the sham group (1.20 ± 0.09 vs 2.13 ± 0.14 µmol/100 mg tissue, respectively; P < 0.05). However, EPO significantly decreased MDA content (1.56 ± 0.1 µmol/100 mg tissue; P < 0.05) compared to the IR group. Ischemic preconditioning also significantly decreased MDA content (1.54 ± 0.07 µmol/100 mg tissue P < 0.05) compared to the IR group (Figure 3A).
The sham group did not undergo any further interventions; the ischemia/reperfusion (IR) group was subjected to 50 minutes bilateral ischemia followed by 24 hours reperfusion. A single dose of EPO (5000 U/kg, i.p) was administered 30 minutes prior to ischemia. Ischemic preconditioning consisted of three cycles of 3 minutes intermittent IR of renal pedicles applied at the beginning of the 50 minutes period of renal ischemia, followed by 24 hours reperfusion. Data are the mean ± SEM (n = 6 to 9). *P < 0.05 compared to the sham group; #P < 0.05 compared to the IR group (one-way ANOVA followed by Tukey’s test).
In addition, bilateral renal ischemia (50 minutes) and 24 hours reperfusion significantly decreased SOD activity compared to the sham group (29.84 ± 2.78 vs 9.04 ± 1.44 U/g tissue, respectively; P < 0.05). The SOD activity in the EPO group (22.37 ± 2.34 U/g tissue; P < 0.05) was significantly higher than that in the IR group. Ischemic preconditioning also significantly increased the SOD activity (21.48 ± 3.22 U/g tissue; P < 0.05) compared to the IR group (Figure 3B).
Compared to the sham group, bilateral renal ischemia (50 minutes) and 24 hours reperfusion resulted in a significant decrease in GSH levels (79.63 ± 3.5 vs 26.02 ± 4.36 µmol/g tissue, respectively; P < 0.05). The GSH level was increased in the EPO group (67.69 ± 4.48 µmol/g tissue P < 0.05) compared to that in the IR group. The GSH levels were also increased in the IPC group (64.74 ± 3.42 µmol/g tissue; P < 0.05) compared to that in the IR group (Figure 3C).
A summary of the results are given in Table 1.
| Biochemical and Oxidative Stress Indices | Groups | |||
|---|---|---|---|---|
| Sham | IR | EPO | IPC | |
| BUN, mg/dL | 22.50 ± 3.18 | 133.33 ± 5.48b | 64.33 ± 8.75 | 68.60 ± 5.87 |
| sCr, mg/dL | 0.29 ± 0.08 | 2.75 ± 0.40b | 1.26 ± 0.36c | 1.28 ± 0.14c |
| MDA, µmol/100 mg Tissue | 1.20 ± 0.09 | 2.13 ± 0.14b | 1.56 ± 0.1c | 1.54 ± 0.07c |
| SOD, U/g Tissue | 29.84 ± 2.78 | 9.04 ± 1.44b | 22.37 ± 2.34c | 21.48 ± 3.22c |
| GSH, µmol/g Tissue | 79.63 ± 3.5 | 26.02 ± 4.36b | 67.69 ± 4.48c | 64.74 ± 3.42c |
aValues are expressed as mean ± standard error of the mean.
bP < 0.05 compared to the sham group.
cP < 0.05 compared to the IR group.
5. Discussion
Renal IR injury is a complicated process in which the kidney is subjected to morphological and functional damage during the ischemic phase and undergoes further insult during reperfusion. In the present study, we used a rat model of renal IR injury (50 minutes) bilaterally. The effect of pretreatment with EPO or IPC on renal function, as well as on MDA (a marker of lipid peroxidation) and SOD and GSH (markers of antioxidants) were investigated. In this study, we present the finding that EPO pretreatment and application of IPC protect the kidney against IR injury induced by 50 minutes bilateral ischemia followed by 24 hours reperfusion. Our study show that EPO pretreatment and application of IPC reduce renal dysfunction (assessed by BUN and creatinine), oxidative stress (assessed by SOD and GSH), and lipid peroxidation (assessed by MDA).
The present study demonstrated that 50 minutes bilateral renal IR injury caused significant increases in plasma levels of BUN and creatinine. These findings confirm that IR injury of the kidney causes both glomerular and tubular dysfunctions and are in agreement with those reported by Gobe et al. (14).
The sequential events of renal IR injury include both cellular damage caused by ischemic insult and generation of reactive oxygen species which results in activated vascular endothelial cells after reperfusion (15) that cause renal cell injury. To dismiss toxic reactive oxygen species, cells have many natural defense enzyme mechanisms, including the enzymes SOD and CAT and the anti-oxidant molecule GSH. An increase in free radical causes overproduction of ROS during IR which may lead to the consumption and depletion of these endogenous scavenger antioxidants. In the present study, SOD activity was significantly lower in the IR group compared to the sham group. Depletion of GSH was also seen in the IR group. These observations are in accordance with previous studies that reported that renal IRI is associated with decreases in SOD activity (16). Depletion of GSH content is also in agreement with previous findings (17). Our results demonstrated that, pretreatment with EPO as a single dose or application of IPC, significantly increased SOD activity and GSH content. In agreement with these findings Baranano and Snyder (18) reported that EPO increased the production of radical scavengers, and Akisu et al. (19) showed that EPO inhibited the iron-catalyzed reactions for generating free oxygen radicals. The data of another study showed that EPO pretreatment improved the cellular antioxidant defense system following renal IR injury (20).
Tissue MDA content is one of the most known indicators of lipid peroxidation. Previous studies have demonstrated that, MDA tissue content is also commonly used as a marker of oxidative stress in renal IR injury (21). In the present study, we demonstrated that IR resulted in increased MDA content in renal tissues and was associated with impaired kidney function. These data are in good agreement with those of Jiang et al. who found high lipid peroxidation after renal IR injury (22). Our results demonstrated that pretreatment with EPO significantly decreased the level of MDA, indicating lower level of oxidative stress and subsequently less lipid peroxidation. Consistent with our findings, Ates et al. (23) demonstrated that EPO decreased the level of MDA after IR injury in a rat model. Our results showed that application of IPC caused a significant reduction in MDA production, indicating a reduction in lipid peroxidation and cellular damage. Consistent with our findings, Ahmed et al. (24) demonstrated that, IPC significantly reversed the increase in lipid hydroperoxide levels to a considerable extent.
Renal IR damage leads to extensive oxidative stress. Reducing the excessive production of reactive oxygen species minimizes the secondary destruction after renal injury (25). Erythropoietin may exert its anti-oxidative effects by stimulating endothelial nitric oxide synthase and inducing intracellular anti-oxidative mechanisms. Plentiful of reactive oxygen species during the first stage of reperfusion has been presented out as the good evidence for the pathogenesis of the tissue injury. Reactive oxygen species generation increases sharply at the onset of reperfusion, although such substances are detectable in further moments too (26). The IPC acts in this phase in a way that has not been completely clear, probably attenuating the oxidative stress through increased production of nitric oxide. A limitation of the current study is the lack of long-term evaluation of the kidney function of the experimental groups. Another limitation is the lack of some groups to measure the parameters in the middle of the reperfusion period.
In conclusion, pretreatment with EPO and application of IPC significantly ameliorated the renal injury induced by bilateral renal IR. However, both treatments attenuated renal dysfunction and oxidative stress in kidney tissues. There were no significant differences between pretreatment with EPO or application of IPC.