1. Background
Protein-energy wasting (PEW) (or malnutrition as it was previously called) is a common and severe complication in patients with end-stage renal disease. Protein-energy wasting and heightened inflammation are highly prevalent in patients undergoing continuous ambulatory peritoneal dialysis (CAPD) and is a strong risk factor for morbidity and mortality in these patients (1-3).
Although PEW is not the direct cause of death in the majority of patients undergoing peritoneal dialysis (PD), the presence of nutritional abnormalities is likely a marker of comorbid illness that may lead to adverse outcomes.
Periodic measurement of C-reactive protein (CRP) or Interleukin-6 (IL-6) to assess severity and frequency of inflammation is reasonable in patients with protein-energy wasting as part of individualized workup. Although it may improve patient’s management, routine monitoring of inflammatory markers in all patients with end-stage renal disease (ESRD) is currently not recommended by most guidelines. As a result, evaluation of protein-energy wasting by various methods, prevalence of inflammation, as well as interrelationship between various nutritional indices and inflammation have not been studied in much detail in patients undergoing CAPD.
2. Objectives
This study was undertaken to evaluate the interrelationship between PEW and inflammation in patients undergoing CAPD.
3. Patients and Methods
A total of 63 patients undergoing CAPD (3 months after its initiation) were selected from nephrology outpatient department. Calorie intake (kg/d), protein intake (d), high biological value protein intake, carbohydrate intake, and fat intake (d) were calculated in our patients from the average of 3-day dietary diary (food-intake record). Also, the following anthropometrical measurements such as body weight (BW), body mass index (BMI), Tricipital skin-fold thickness (TST), and mid-arm circumference (MAC) were performed. The mid-arm muscle circumference (MAMC) and mid-arm muscle area (MAMA) were derived from MAC and TST. By using the multifrequency bioelectrical impedance analysis (BIA) machine, we directly measured the different body composition. The lean tissue index (LTI) and fat tissue index (FTI) values of the patients were displayed on the screen along with the upper and lower limits of the normal values of that patient. Adipose tissue mass, fat, lean tissue mass, body cell mass and phase angle were noted too. Subjective global assessment (SGA) was based on history and physical examination, according to the method described by Detsky, and the results were recorded (4).
Various serological markers like blood urea, hemoglobin, serum creatinine, cholesterol, albumin, triglycerides, and electrolytes were measured. Serum prealbumin, transferrin, and hs-CRP were measured by immunoturbidimetry method. Serum IL-6 was measured by photometric ELISA method. Patients undergoing CAPD were assessed for the protein-energy wasting and diagnosed accordingly by the following criteria. Based on SGA, patients were graded as severe, mild to moderate, or well nourished if they got 6 to 7 ratings, 3 to 5 ratings, or 1 to 2 ratings, respectively in most categories (4). Also, patients were categorized as in well, mild, or severe malnutrition, if their serum albumin levels were > 3.5 g/dL, 2.5 - 3.5 g/dL, or < 2.5 g/dL, respectively. Likewise, the patients were categorized as in well, mild, and severe malnutrition, if their BMI were > 23, 18.5 - 23, or < 18.5, respectively (5). Finally, the patients with hs-CRP > 3mg/L and IL-6 > 2 µg/mL were diagnosed as having high inflammation. In continuation, the correlation between inflammation and protein-energy wasting was analyzed.
For statistical analysis, SPSS for Windows (version 19.0) was used. Parametric data were described as mean ± standard deviation. Significant association between continuous variables was described using Pearson’s and Spearman’s correlation coefficient for parametric and nonparametric data, respectively. A P value of less than 0.05 was considered statistically significant.
4. Results
A total of 63 patients (28 men and 35 women) were included in the study. The age of the patients ranged from 22 to 79 years with a mean age of 57.6 years. Among 63 patients in our study, the causes for ESRD were diabetic nephropathy in 28 patients (44%) and hypertensive nephrosclerosis in 14 patients (22%). Totally, 30 episodes of peritonitis were observed in 1042 months duration (1 in 34.7 patient-treatment-month). The average calorie and protein intake per kg/d were 25.4 kcal and 0.81 g, respectively. The average protein intake per day and high biological protein intake was 47.9 g and 21.7 g, respectively and the latter was less than 50%. The mean and standard deviation of various anthropometry and serological markers were shown in Table 1.
| Parameters | Male (28) | Female (35) | Total (63) |
|---|---|---|---|
| Total Calorie Intake, kcal /d | 1438.3 ± 223.6 | 1510.6 ± 193.9 | 1478.4 ± 209.0 |
| Calorie intake, kcal/kg/d | 25.5 ± 4.6 | 25.4 ± 4.6 | 25.4 ± 4.6 |
| Total protein intake, g/d | 45.2 ± 13.1 | 50.1 ± 10.6 | 47.9 ± 12.0 |
| Protein intake, g/kg/d | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.81 ± 0.2 |
| High biological protein intake, g/d | 20.6 ± 7.0 | 22.8 ± 6.0 | 21.7 ± 6.7 |
| Carbohydrate intake, kcal/d | 846.5 ± 162.5 | 905.1 ± 149.6 | 879.1 ± 156.9 |
| Fat intake, kcal/d | 359.6 ± 55.7 | 355.1 ± 61.2 | 357.1 ± 58.4 |
| Weight, kg | 57.5±10.1 | 60.9±11.8 | 59.4±11.1 |
| Height, m | 1.7±0.1 | 1.5±0.1 | 1.6±0.1 |
| Body mass index, kg/m2 | 21.1±3.3 | 25.8±5.1 | 23.7±5.0 |
| Mid-arm circumference, cm | 25.05±4.2 | 27.32 ± 4.5 | 26.3 ± 4.5 |
| Tricipital skin-fold thickness, cm | 1.48 ± 0.4 | 1.74 ± 0.4 | 1.624 ± 0.4 |
| Mid-arm muscle circumference, cm | 24.4 ± 4.2 | 26.5 ± 4.5 | 25.6 ± 4.5 |
| Corrected mid-arm muscle area, cm | 39.0 ± 17.5 | 51.0 ± 20.0 | 45.7 ± 19.7 |
| Haemoglobin, g/dL | 10.6 ± 1.7 | 10.4 ± 1.5 | 10.5 ± 1.6 |
| Blood urea, mg/dL | 100.9 ± 28.9 | 88.8 ± 28.3 | 94.2 ± 29.0 |
| Serum creatinine, mg/dL | 6.7 ± 2.4 | 6.4 ± 1.7 | 6.5 ± 2.0 |
| Serum potassium, mg/dL | 4.5 ± 0.5 | 4.4 ± 0.5 | 4.5 ± 0.5 |
| Serum phosphorus, mg/dL | 5.0 ± 1.0 | 4.6 ± 1.2 | 4.7 ± 1.1 |
| Serum protein, g/dL | 5.8 ± 0.6 | 6.0 ± 0.7 | 5.9 ± 0.7 |
| Serum albumin, g/dL | 2.9 ± 0.4 | 3.0 ± 0.5 | 3.0 ± 0.5 |
| Serum prealbumin, mg/dL | 20.57 ± 5.90 | 21.54 ± 8.95 | 21.11 ± 7.70 |
| Serum transferrin, mg/dL | 129.07 ± 36.50 | 137.57 ± 37.62 | 130.6 ± 39.70 |
| Serum HCO3, mmol/L | 19.5 ± 2.7 | 19.57 ± 2.9 | 19.54 ± 2.8 |
| Serum cholesterol, mg/dl | 141.4 ± 33.8 | 167.5 ± 39.2 | 155.9 ± 38.9 |
| Serum triglycerides, mg/dl | 126.8 ± 26.9 | 143.4 ± 38.3 | 136.1 ± 34.5 |
| Lean tissue index, kg/m2 | 10.0 ± 2.0 | 9.3 ± 2.7 | 9.6 ± 2.4 |
| Fat tissue index, kg/m2 | 7.4 ± 4.1 | 12.5 ± 3.8 | 10.2 ± 4.6 |
| Lean tissue mass, kg | 27.5 ± 6.5 | 22.0 ± 6.4 | 24.5 ± 7.0 |
| FAT, kg | 20.3 ± 11.2 | 29.4 ± 8.6 | 25.4 ± 10.8 |
| Adipose Tissue Mass, kg | 24.9 ± 11.5 | 34.4 ± 8.9 | 30.1 ± 11.1 |
| BCM, kg | 15.9 ± 4.5 | 12.8 ± 4.3 | 14.2 ± 4.7 |
| Phase angle | 3.6 ± 0.8 | 3.63 ± 0.6 | 3.619 ± 0.7 |
| hs-CRP, mg/L | 8.7 ± 8.3 | 8.9 ± 7.2 | 8.8 ± 7.6 |
| IL-6, µg/mL | 10.6 ± 15.7 | 6.6 ± 8.4 | 8.4 ± 12.2 |
aData are presented as mean ± SD.
The mean values of hs-CRP and IL-6 were 8.8 mg/L and 8.4 µg/mL, respectively, which were in the higher range in the study population. The mean LTI (10 kg/m2) values were high in males, while mean FTI (12.5 kg/m2), ATM (34.4 kg), and FAT (29.4 kg) values were higher in females as compared to males. The mean phase angle of males and female were 3.6 and 3.63, respectively.
4.1. The Prevalence of Protein-Energy Wasting Based by Various Methods
4.1.1. Anthropometry
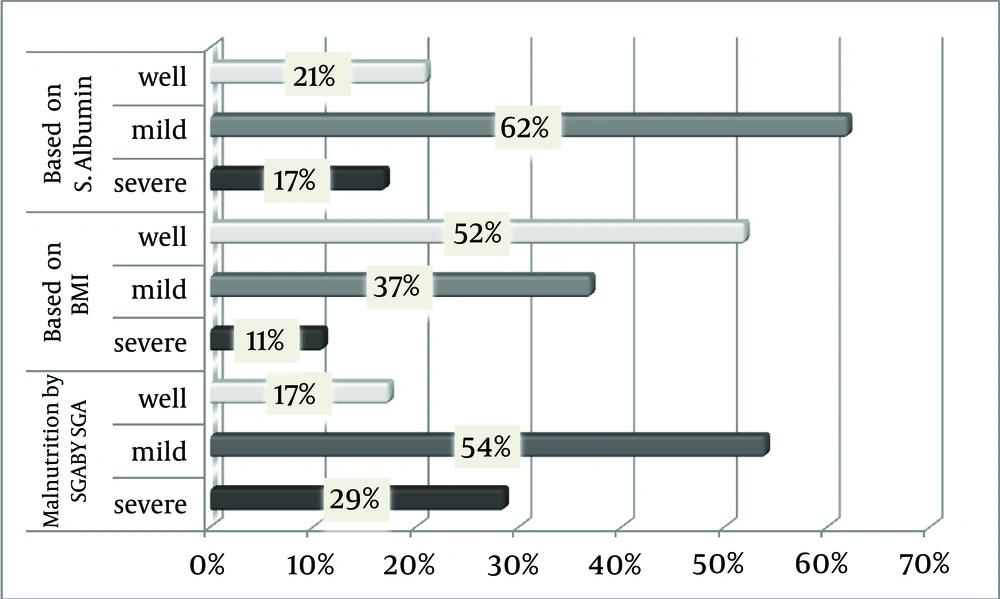
About 47.6% (11.1% + 36.5%) of patients undergoing CAPD (severe and mild) had BMI < 23 kg/m2. Majority of females had BMI > 23 (27 out of 35) while most of the males had BMI less than 23 (22 out of 28). This may be due to the presence of more fat in females. About 32.1% (9 out of 28) of males and 20% (7 out of 35) of females were malnourished according to MAC. The prevalences of protein-energy wasting based on various methods are shown in Figure 1.
4.1.2. Bioelectrical Impedance Analysis
Based on normal range shown in the multifrequency BIA, malnourishment can be diagnosed, if the values of LTI and FTI lie below the lower limit of the normal range. A total of 48 out of 63 patients in the study group (76.1%) were malnourished based on LTI. And based on FTI, 6 out of 63 patients in the study group (9.5%) were malnourished.
4.1.3. Subjective Global Assessment
About 83 % of patients in the study group were malnourished based on SGA.
4.1.4. Serology
Majority of the study population (83 %) had serum albumin < 3.5 g/dL (11 out of 63). Based on serum prealbumin (< 30 mg/dL), 92% of the study population (58 out of 63) were malnourished. Based on serum transferrin (< 200 mg/dL), 92% of the study population (58out of 63) were malnourished. Also based on serum cholesterol (< 100 mg/dL), 9.5% of the study population (6 out of 63) were malnourished.
4.2. Markers of Inflammation
Patients with hs-CRP > 3 mg/L and IL-6 > 2 µg/mL were diagnosed as having high inflammation. Based on hs-CRP, 70 % of patients undergoing CAPD (44 out of 63) and based on IL-6, 71.8% of the patients (45 out of 63) had high inflammation status.
4.3. Correlation of Inflammatory Markers With Other Nutritional Parameters
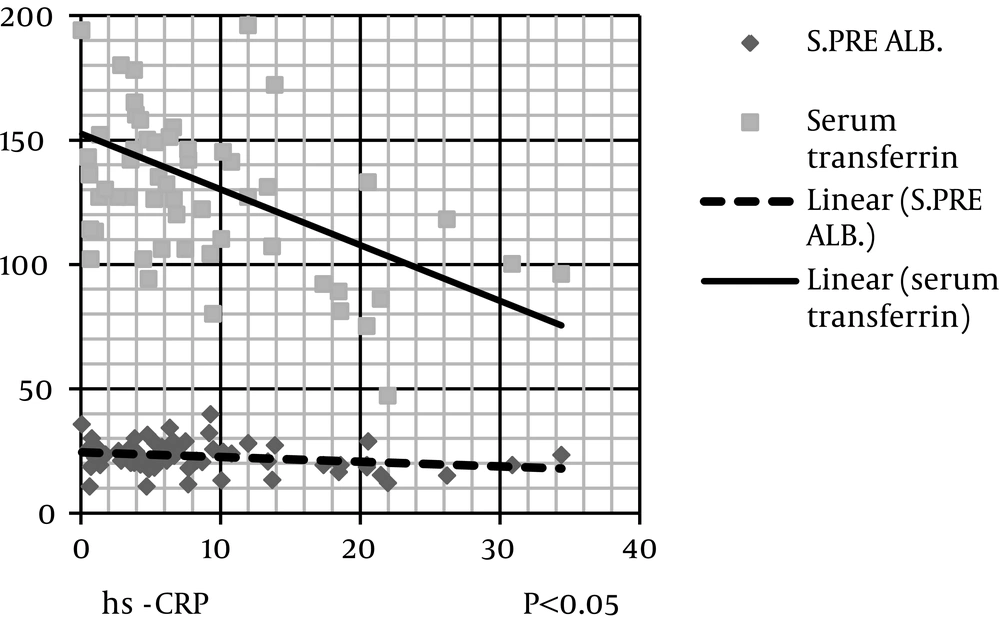
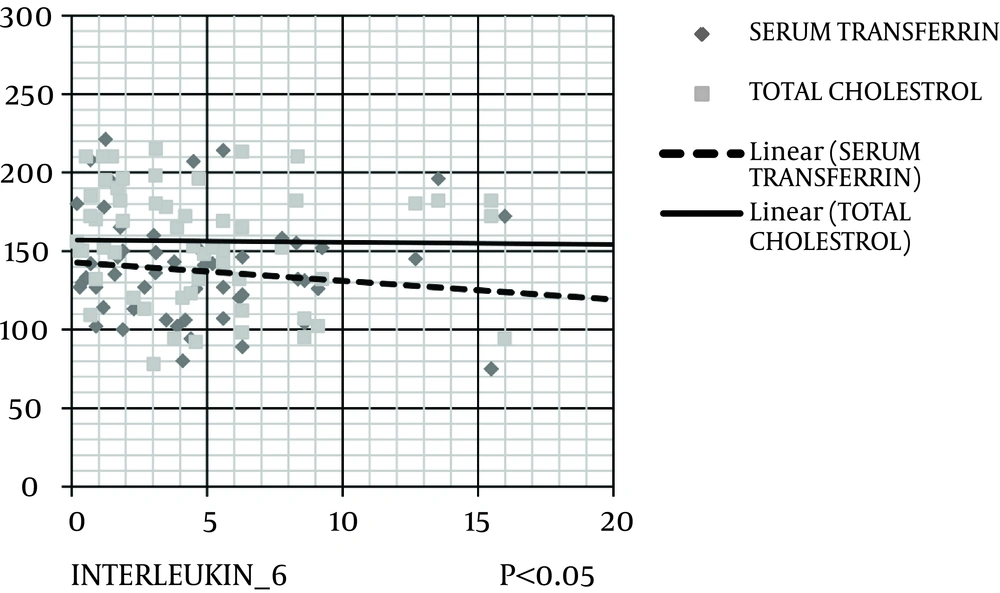
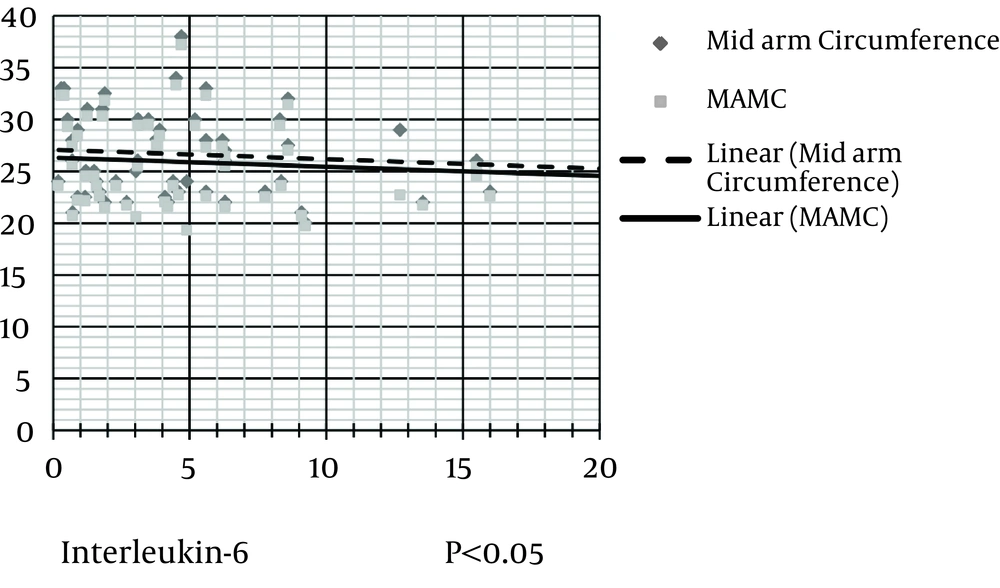
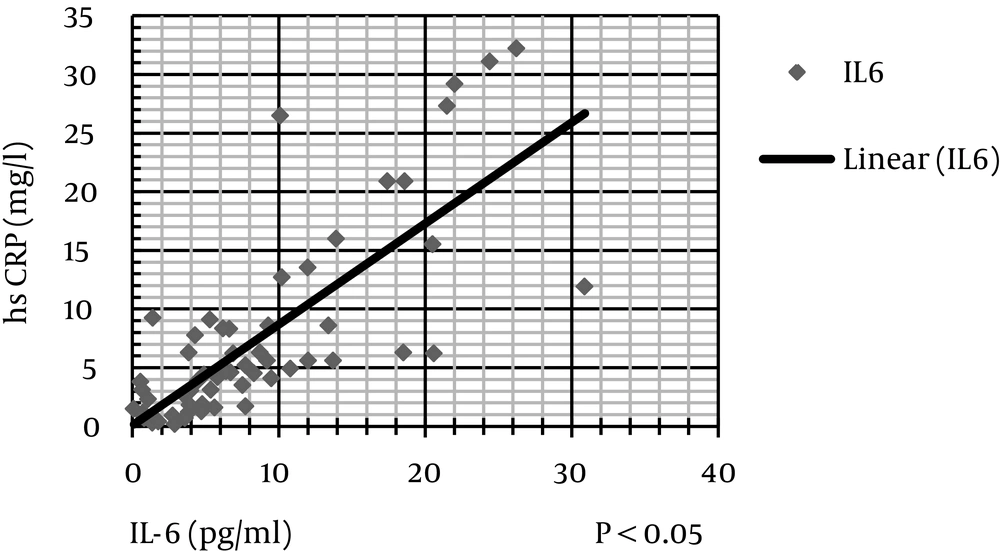
The level of hs-CRP was correlated negatively and significantly with protein (kg/d), serum albumin, serum prealbumin, and serum transferrin (Figure 2). IL-6 level was correlated negatively and significantly with protein (kg/d), MAC, MAMA, serum albumin, serum cholesterol, and serum transferrin (Figures 3 and 4). IL-6 level was correlated positively and significantly with hs-CRP level (Figure 5).
Scatter dot diagram (Figure 2) shows that hs-CRP level negatively and significantly correlates with serum prealbumin and serum transferrin (Figure 3). IL-6 level negatively and significantly correlates with serum cholesterol and transferrin levels (Figure 4). In addition, IL-6 level significantly and negatively correlates with MAC and MAMC values (Figure 5). Finally, IL-6 correlates positively with hs-CRP level.
Correlations of inflammatory markers with other nutritional parameters are shown in Table 2. The means and standard deviations of various nutritional parameters of patients with and without inflammation and their significances are shown in Table 3. There is statistically significant difference in total protein intake g/d, protein intake g/kg/d, serum protein (g/dL), serum albumin (g/dL), serum transferrin (mg/dL), and serum cholesterol (mg/dL) between patients with and without inflammation.
| Parameters | hs-CRP | IL-6 | ||
|---|---|---|---|---|
| P | Test Result | P | Test Result | |
| Calorie intake kcal, kg/d | .46 | NA | .23 | NA |
| Protein, kg/d | .02a | R = -0.28 | .03a | R = -0.28 |
| Body mass index, kg/m2 | .84 | NA | .63 | NA |
| Mid-arm circumference, cm | .39 | NA | .04 b | rs = -0.25 |
| Tricipital skin-fold thickness, cm | 1.00 | NA | .10 | NA |
| Mid-arm muscle circumference, cm | .27 | NA | .04b | rs = -0.26 |
| Corrected mid-arm muscle area, cm | .36 | NA | .053 | NA |
| Serum phosphorous, mg/dL | .11 | NA | .79 | NA |
| Serum albumin, gm/dL | .0006a | R = -0.42 | .0498a | R = -0.25 |
| Serum prealbumin, mg/dL | .04a | R = -0.26 | .44 | |
| Serum transferrin, mg/dL | .001a | R = -0.39 | .004a | R = -0.36 |
| Serum bicarbonate, mmol/L | .44 | NA | .11 | NA |
| Serum cholesterol, mg/dL | .22 | NA | .01a | R = -0.34 |
| hs-CRP, mg/L | 1.0 | NA | < .000001b | rs = 0.7 |
| Kt/V | .51 | NA | .55 | NA |
aR = Pearson coefficient.
bSpearman constant.
Abbreviations: hsCRP, high sensitivity C-reactive protein; Kt/V, dialysis Adequacy; NA, Not available.
| Parameters | Patients Without Inflammation | Patients With Inflammation | P Value | |
|---|---|---|---|---|
| Mann-Whitney test | T test | |||
| Total Calorie Intake, kcal /d | 1585.72 ± 138.00 | 1435.51 ± 218.19 | NA | .17 |
| Total protein intake, g/d | 52.56 ± 13.14 | 46.05 ± 11.08 | NA | .049 |
| Protein intake, g/kg/d | 0.89 ± 0.17 | 0.78 ± 0.14 | NA | .01 |
| Body mass index, kg/m2 | 23.72 ± 4.54 | 23.68 ± 5.16 | .95 | NA |
| Mid-arm circumference, cm | 26.81 ± 4.14 | 26.11 ± 4.61 | .44 | NA |
| Tricipital skin-fold thickness, cm | 1.68 ± 0.43 | 1.60 ± 0.43 | .45 | NA |
| Mid-arm muscle circumference, cm | 26.28 ± 4.02 | 25.30 ± 4.63 | .30 | NA |
| Corrected mid-arm muscle area, cm | 47.95 ± 17.32 | 44.74 ± 20.70 | .37 | NA |
| Blood haemoglobin, gm/dL | 10.86 ± 1.45 | 10.14 ± 1.59 | NA | .10 |
| Blood urea, mg/dL | 99.33 ± 29.81 | 92.08 ± 28.68 | .48 | NA |
| Serum creatinine, mg/dL | 6.27 ± 2.11 | 6.62 ± 2.01 | NA | .55 |
| Serum potassium, mg/dL | 4.49 ± 0.53 | 4.53 ± 0.54 | NA | .79 |
| Serum phosphorous, mg/dL | 4.88 ± 1.29 | 4.70 ± 1.05 | NA | .56 |
| Serum protein, g/dL | 6.18 ± 0.57 | 5.81 ± 0.69 | NA | .04 |
| Serum albumin, g/dL | 3.20 ± 0.41 | 2.88 ± 0.46 | .03 | NA |
| Serum prealbumin, mg/dL | 23.12 ± 6.53 | 22.64 ± 6.16 | NA | .78 |
| Serum transferrin, mg/dL | 149.67 ± 34.70 | 126.24 ± 34.85 | NA | .02 |
| Serum HCO3, mmol | 20.12 ± 2.65 | 19.31 ± 2.81 | .27 | NA |
| Serum cholesterol, mg/dL | 171.50 ± 28.45 | 149.64 ± 40.93 | NA | .04 |
| Serum triglycerides, mg/dL | 139.06 ± 27.98 | 134.84 ± 36.94 | NA | .66 |
| Lean tissue index, kg/m2 | 9.03 ± 2.11 | 9.86 ± 2.53 | .36 | NA |
| Fat tissue index, kg/m2 | 11.19 ± 4.95 | 9.87 ± 4.49 | NA | .31 |
| Lean tissue mass, kg | 22.88 ± 6.82 | 25.08 ± 7.01 | NA | .26 |
| FAT | 27.83 ± 12.72 | 24.41 ± 9.86 | NA | .26 |
| Phase angle | 3.71 ± 0.61 | 3.58 ± 0.72 | NA | .50 |
aData are presented as mean ± SD.
Abbreviation: NA, Not available.
5. Discussion
Currently, peritoneal dialysis is an emerging modality of Renal Replacement Therapy (RRT). Nutritional status of patients undergoing peritoneal dialysis (PD) is an important predictor of their clinical outcome.
In this study, the mean (SD) age of the patients was 57.6 (11.6) years out of which 55.5% were females. Diabetic nephropathy was the major cause (44.4%) of ESRD in the study sample, and the prevalence of CAD in diabetic patients was 60.7%. Nearly 63 % of the study population had high and above-average transport which explains the high prevalence of malnutrition and inflammation in our study. High peritoneal transport status in the incident of patients with PD in particular has received increased attention due to poor outcome, higher prevalence of markers of inflammation, malnourishment, and/or hypoalbuminemia (6). The average calorie and protein intake were very low in the study population too. The mean (SD) calorie intakes (kcal/kg/d) of patients aged < 60 y and aged > 60 y were 25.45 (4.52) and 25.43 (4.65), respectively as against the K/DOQI (kidney disease outcomes quality initiative) guidelines recommendation. In this study, only 15.7% of patients took more than 30 kcal/kg/d. The mean protein intake of patients was 0.81 g/kg/d. Only 12.6% of patients took more than 1 g/kg/d. Even though the mean calorie intake in the study group was higher , the high biological value protein intake was lower as compared to that in the study by Prasad et al. (7) which explains the higher prevalence of malnutrition in our study group (1478 kcal vs 1089 and 21.7 vs 24.26 g/d).
5.1. Malnutrition Grading Based on Subjective Global Nutritional Assessment
In this study, 54% of the patients had mild to moderate, and 28.6% had severe malnutrition. The prevalence of malnutrition in our patients undergoing CAPD appears to be very high compared to reports from other cross-sectional studies. Many multicenter studies also estimate that severe malnutrition ranges from 4% to 8%, and mild to moderate malnutrition from 33% to 55% (7-13). Similar to our study, Naicker (14) reported a very high prevalence of malnutrition (76.2%) in the studied patients undergoing PD, with loss of appetite as an important etiological factor. Also Prasad et al. (7) reported prevalence of malnutrition (74.91%) in Indian population. With regard to serological investigations, about 61.9% of the study population had serum albumin level between 2.5 to 3.5 g/dL. The large-scale prevalence data from the CANUSA (Canada-USA) study showed that 55% of patients had some degree of malnutrition, whereas 4.2% were severely malnourished, as determined by serum albumin and SGA (3). Regarding anthropometry findings, in 52.3 % of the study population, BMI was above 23. In this study, BMI cut off was taken as 23 instead of 18.5 (recommended by WHO criteria) because Kalantar et al. (5) observed increased mortality in Dialysis patients whose BMI is less than 23. As per BMI criteria, only 47.7% of our patients were malnourished as against 83.6%, and 79.4% malnourished patients based on SGA and serum albumin level, respectively. This shows that BMI is not a sensitive indicator for malnutrition as it is influenced by overhydration, which is common in ESRD patients. The mean (SD) mid-arm circumference and mid-arm muscle circumference were 26.3 (4.5) and 25.6 (4.5) cm, respectively. These values were lower as compared to that in previous study by Wolfson et al. (15) which were 28.9 (3.6) and 26.7 (3.0) cm, respectively in patients on dialysis.
5.2. Markers of Inflammation
In this study, the mean (SD) value of hs-CRP was 8.8 (7.6) mg/L and this value was higher as compared to that value in a previous study by Ducloux et al. (16), who reported initial median serum CRP level of 7 mg/L in a prospective report of 240 patients undergoing peritoneal dialysis. The mean (SD) value of IL-6 was found to be 8.4 (12.2) µg/mL in the study group, which was higher as compared to that value in a previous study by Pecoits et al. (17), who reported the mean serum IL-6 concentrations among patients undergoing peritoneal dialysis as 5.6 µg/mL. In this study, the mean hemoglobin level among patients with high degree of inflammation was found to be 10.1 g/dL which was lower as compared to 10.9 g/dL in patients without evidence of inflammation. This may be explained by erythropoietin hyporesponsiveness which is believed to be seen in malnutrition-inflammation complex syndrome (MICS) (18). This syndrome leads to low BMI, hypocholesterolemia, hypocreatininemia, also a “reverse epidemiology” of cardiovascular risks can occur in dialysis patients (18). This condition can explain the low mean values of serum cholesterol and serum creatinine among patients with high degree of inflammation as compared to patients without evidence of inflammation (170 mg/dL vs 152 mg/dL and 6.5 vs 6.4 mg/dL, respectively). In this study, it was observed that there was increased serum levels of hs-CRP in 84% of patients (21 out of 25) with past h/o peritonitis. In a multicenter cross-sectional study on 183 patients undergoing PD, Avila-Diaz et al. (19) reported the prevalence of inflammation as 80.3%, (defined as an elevated CRP greater than or equal to 3.0 mg/L), as compared to 70% in our study. Persistent elevations of CRP level are associated with ischemic heart disease in patients undergoing CAPD, also chronic inflammation has been shown to independently contribute to an increased incidence of cardiovascular events in patients undergoing PD (16). Based on these findings, it is useful to incorporate inflammatory markers in the regular assessment of patients undergoing PD, whose survival may be improved by better management of malnutrition and inflammation.