1. Background
Benign prostate hyperplasia (BPH) is a urological disorder caused by the abnormal proliferation of epithelial and stromal cells in the prostate gland in elderly men (1, 2). As the prostate enlarges, it can compress the urethra, inducing various symptoms, such as narrow urine stream, urinary retention, nocturia, dysuria, and bladder outlet obstruction (3). The two main medications used for the treatment of BPH are α-blockers and 5α-reductase inhibitors (4). The α-blockers relax the smooth muscles in the prostate (5) and the 5α-reductase inhibitors reduce the rate of conversion of testosterone into dihydrotestosterone (DHT), which accelerates hyperplasia of the stromal and epithelial cells of the prostate, causing prostate enlargement (6). However, the most common side effects of these drugs include dizziness, diarrhea, orthostatic hypotension, headache, nasal congestion, and sexual dysfunction (7).
Due to the many side effects of drug therapy, phytotherapy is becoming more accepted and is frequently used to alleviate the symptoms of BPH (8). Withania coagulans belongs to the family Solanaceae and is an important medicinal plant commonly used in Iran, Pakistan, Afghanistan, and East India (9). W. coagulans extract (WCE) has been reported to possess antimicrobial, anti-inflammatory, hepato-protective, antihyperglycemic, cardiovascular, and free-radical scavenging properties (10). WCE has also shown antiproliferative effects and is traditionally used for the treatment of cancerous tumors (11).
Withaferin-A (WA) is a steroidal lactone and a major constituent of the Withania plant. It exhibits an inhibitory effect against several different types of cancer cells, including those of prostate cancer (12). Some studies have shown that WA inhibits the growth of human cancer cells by causing apoptosis (13).
2. Objectives
Although many researchers have investigated the pharmacological effects of W. coagulans, there has been no study on its possible protective effects against BPH. Therefore, we investigated the antiproliferative and antioxidant effects of WCE on benign prostatic hyperplasia in rats.
3. Materials and Methods
3.1. Preparation of Plant Extract
W. coagulans (WC) plants were collected from the herbarium center of Sistan and Baluchestan university in January 2015. The air-dried whole plants were ground into a fine powder. A mixture of methanol and water (20 mL, 3:1 v/v) was added to the WC powder (1 g) and the mixture was kept at room temperature overnight. The extracts were filtered, concentrated by removing excess solvent with a rotatory flash evaporator, and completely freeze-dried. They were then stored in airtight containers under refrigeration.
3.2. Animals
A total of 40 male Wistar rats, aged 3 months and weighing 200 - 250 grams, were supplied by the animal lab of Zahedan University of Medical Sciences. The rats were kept in standard cages in a controlled temperature (22°C) and light-period (12/12 hours light/dark) environment, with free access to food and water. BPH was induced by subcutaneous injections with 3 mg/kg of testosterone propionate (TP) for four weeks (14). The animals were divided randomly into five groups (each n = 8): the control group, the untreated BPH group, and three WCE groups (250, 500, and 1,000 mg/kg). Animals in all groups received distilled water or WCE by gastric gavage for 28 days in combination with TP, while the control group received 3 mg/kg of corn oil subcutaneously.
At the end of the experiment, the animals were deeply anesthetized with chloroform, and after cervical dislocation, their prostates were immediately dissected out and weighed. Half of the prostate was fixed in 10% neutral-buffered formalin and embedded in paraffin for histological analysis, and the other half was stored at −80°C for biochemical assays.
All animal procedures were performed according to the guidelines of the institutional ethics committee of Zahedan medical university (EC/93/7149).
3.3. Prostate Index Calculation
The prostate index of each rat was calculated by dividing prostate weight into body weight (mg/g) and multiplying by 100 (1).
3.4. Biochemical Analysis
3.4.1. Preparation of Prostate Homogenates
Prostate tissue was homogenized (1/10 w/v) in tissue lysis/extraction reagent containing protease inhibitors. Homogenates were centrifuged at 12,000 g for 25 minutes at 4°C. The content of protein in the supernatant fractions was determined by the method of Bradford (15).
3.4.2. Malondialdehyde (MDA) Assay
Lipid peroxidation was estimated spectrophotometrically using the thiobarbituric acid reactive substance (TBARS) method, which measures the malondialdehyde (MDA) level, as described by Ohkawa et al. (16). This method was used to spectrophotometrically measure the color produced by the reaction of thiobarbituric acid (TBA) with MDA. Briefly, 0.5 mL of 10% homogenate, 3 mL of 1% phosphoric acid, and 1 mL of 6% TBA solution were mixed in a 10 mL centrifuge tube. The mixture was heated in a boiling water bath for 45 minutes; after cooling, 4 mL of n-butanol was added, and the mixture was shaken vigorously and centrifuged to separate the n-butanol layer. The absorbance of the pink-colored product was read at 535 nm. 1, 1, 3, 3-tetramethoxypropane was used as an external standard, and the MDA level was expressed as nmol/mg of protein.
3.4.3. Ferric Reducing Antioxidant Power (FRAP) Assay
The total antioxidant capacity of the prostate was determined with the Benzie and strain method (17). A potential antioxidant will reduce the ferric ion (Fe3+) to the ferrous ion (Fe2+), and the latter forms a blue complex (Fe2+/TPTZ), which increases the absorption at 593 nm. Briefly, a working solution of FRAP was produced by mixing 10 volumes of buffer acetate (0.3 M, pH=3.6) with 1 volume of trypyridyl-s-triazine (TPTZ, 10 mM) solution in HCl (40 mM), then 1 mL FeCl3 solution (20 mM/L) was added and mixed. Next, 50 μL of 10% homogenate was added to 1.5 ml of freshly prepared reagent warmed at 37°C for 10 minutes. The complex between Fe2+ and TPTZ gave a blue color with absorbance at 593 nm. FeSO4 was used as a standard for the FRAP assay, and the data were expressed as μmol/g of wet tissue.
3.5. Histological Studies
Ventral prostate tissues were fixed by immersion in 10% formalin-PBS (pH 7.4) at 4°C for 24 hours and processed for paraffin embedding. After dewaxing and rehydration in an ethanol series, sections of 5 μm thickness were stained with hematoxylin and eosin (H&E), then examined under a microscope (Nikon, Tokyo, Japan). Five sections in each group were used for histological examination, and the remainder were used for immunohistochemical examinations.
3.6. Proliferating Cell Nuclear Antigen (PCNA) Staining
Paraffin-embedded tissue sections of 5 µm thickness, taken from four rats per group, were deparaffinized with xylene, then hydrated using an ethanol series and heated in citrate buffer (pH 6.0) for 20 minutes. After that, the sections were blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline (TBS) for 2 hours. The sections were then incubated with mouse monoclonal antibody to PCNA ([PC10] antibody ab29, 1:200) for 2 hours. The slides were washed with TBS, then incubated with the corresponding secondary antibody for 1 hour at room temperature. Staining was developed with diaminobenzidine substrate solution (DAB) and the sections were counterstained with hematoxylin, then they were dehydrated, mounted, and visualized under a light microscope. To determine the percentage of PCNA-positive cells, at least three sections per rat were investigated. The PCNA-staining cells and non-staining cells were counted in different areas of the luminal epithelium, and 500 cells from different tissue sections per animal specimen were analyzed. The percentage of PCNA-positive cells was calculated (number of PCNA-positive cells divided by total number of cells counted, then multiplied by 100) (18, 19).
3.7. Statistical Analysis
The data were analyzed statistically and expressed as the mean ± SEM. Groups were compared using ANOVA followed by Tukey’s test for multiple comparisons. The level of significance was set at P < 0.05.
4. Results
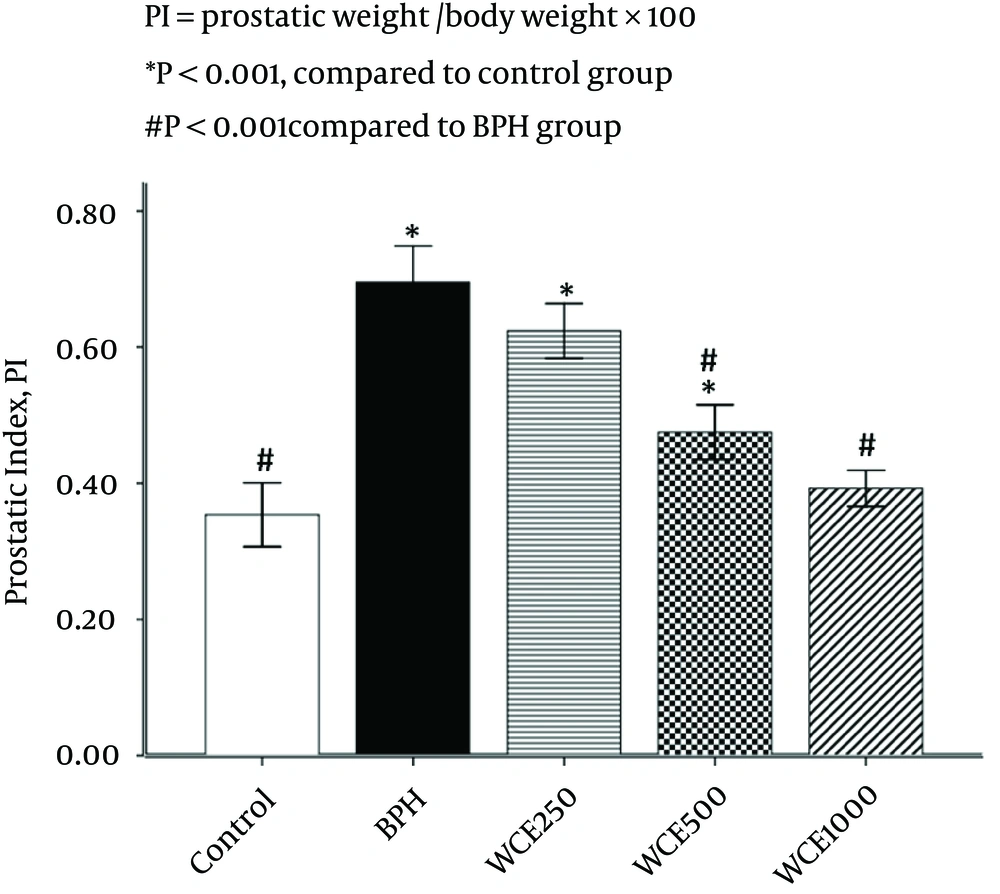
4.1. Effect of WCE on Prostate Index (PI)
Rats in the BPH group showed a significant increase in PI (49.3%) when compared to the control group, whereas PI in the 500 mg/kg and 1000 mg/kg WCE groups was decreased markedly by 32% and 43.5%, respectively, compared with the BPH group (Figure 1). However, WCE 250 mg/kg reduced PI by 10.2% in comparison with the BPH group. There was no significant difference in PI between the control and WCE 1000 mg/kg groups (P > 0.05) (Figure 1).
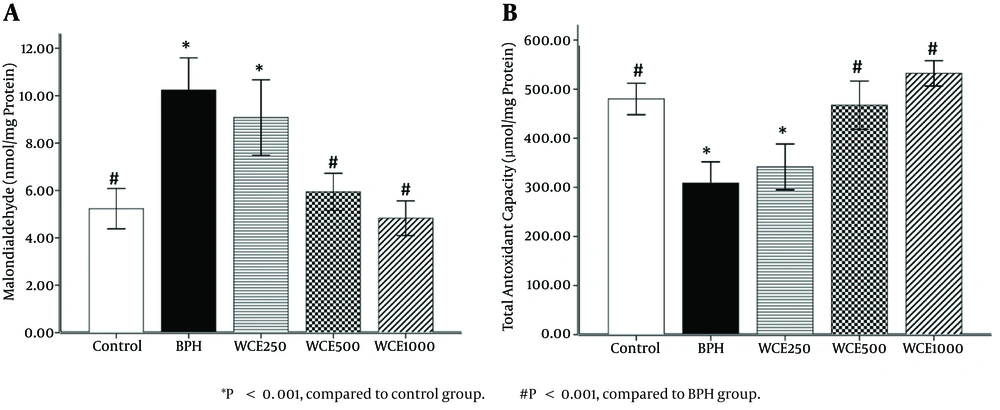
4.2. Effect of WCE on Prostate MDA Levels
The BPH group showed a significant elevation in prostate MDA level (P < 0.001) compared to the control group. On the other hand, the groups treated with WCE showed decreased MDA levels compared to the BPH group. However, the MDA levels of the animals treated with WCE 500 mg/kg or 1000 mg/kg were significantly lower than those in the group treated with WCE 250 mg/kg, when compared to the BPH group (P < 0.001) (Figure 2A).
4.3. Effect of WCE on TAC Levels
A significant decrease (P < 0.001) in TAC levels was observed in the BPH group compared to the control group. Concomitant treatment with WCE 500 mg/kg or 1000 mg/kg significantly increased the TAC level in comparison to the BPH group (Figure 2B). There was no significant difference in the TAC levels between the lower dose of WCE (250 mg/kg) and the BPH group (P > 0.05).
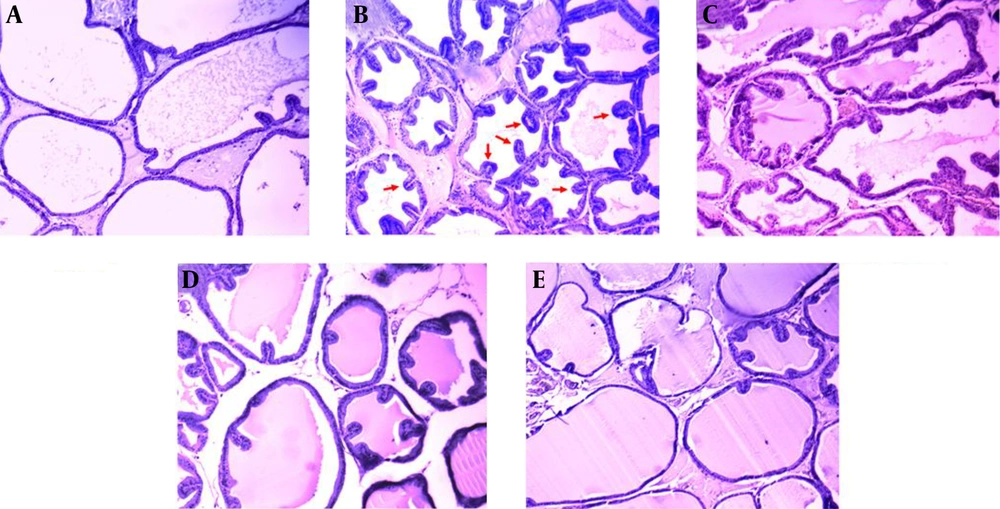
4.4. Histopathological Examination
There were no histopathological alterations in the prostate glands of the control group rats. The epithelium was cuboidal and regular in size (Figure 3A). In contrast in the BPH group, prostatic epithelial cell proliferation had occurred and some papillae had protruded into the acini. Nuclear stratification was also observed (Figure 3B). The epithelium was improved with the highest dose of WCE (1000 mg/kg), more than with the middle or lower doses (500 mg/kg and 250 mg/kg, respectively), when compared with the BPH group (Figure 3C - E).
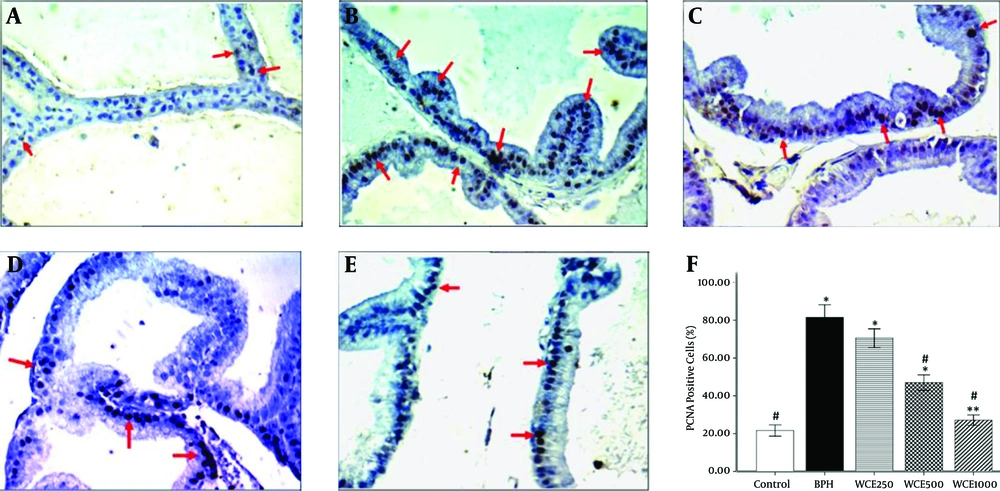
4.5. Proliferating Cell Nuclear Antigen (PCNA)
The immunohistochemistry of the proliferation marker, PCNA, showed a significant increase in the number of stained cells in the BPH group, which indicated an increase in the proliferation rate within this group in comparison to the control group (P < 0.001) (Figure 4B). Concomitant treatment with WCE was able to significantly decrease the number of PCNA-stained cells compared to the BPH group (Figure 4C - E). The number of stained cells in the prostate epithelium of the WCE-treated animals at the highest dose (1000 mg/kg) was significantly lower than in those treated with the middle and lower doses of WCE (500 mg/kg and 250 mg/kg, respectively) (Figure 4F).
A, Control; B, BPH; C, WCE250; D, WCE500; E, WCE1000. Red arrows indicate the PCNA-positive cells (400 × ). The graph shows the percentages of PCNA-positive cells against the total number of cells. *P < 0.001. **P < 0.05, compared to the control group. #P < 0.001, compared to the BPH group.
5. Discussion
The present study investigated the inhibitory effects of W. coagulans on TP-induced BPH in rats. Treatment with WCE for 28 days significantly inhibited the development of BPH, which was observed by increased TAC levels, reduced PI elevations, decreased MDA levels, decreased proliferation markers, and histopathological changes.
Oxidative stress is a factor that plays an important role in prostate hyperplasia (20). It results from an imbalance between free-radical production and free-radical scavenging, and can damage important elements of tissues, such as DNA, lipids, and proteins (21). Some studies have reported that MDA, which is a marker of lipid peroxidation, is elevated in serum samples of BPH patients, clarifying the oxidative stress in these patients (22). Other studies have shown that antioxidant status is decreased in BPH, and management of oxidative stress can prevent the occurrence of BPH (23, 24).
In the present study, MDA levels were found to be significantly increased in the BPH group, and TAC was decreased when compared to the control group. Treatment with WCE dose-dependently and significantly decreased the MDA levels and increased the TAC levels, which is probably due to the antioxidant effects of withanolides, flavonoids, and other components with strong antioxidant potential in W. coagulans (25). In agreement with our study, some evidence has shown that Withania has antioxidant effects by increasing antioxidant levels and decreasing lipid peroxidation in different tissues (26, 27).
BPH, which results from an imbalance between cell proliferation and apoptosis in the prostate gland, can lead to augmentation of the size and weight of the prostate, and consequential partial or complete obstruction of the urethral canal can occur (28). In this study, BPH induced significant increases in the prostate index, which is in accordance with previous studies (19, 29). The WCE-treated animals showed significant decreases in prostate weight. It has been shown that withaferin A, purified from W. coagulans, has antiproliferative effects and stimulates tumor cell apoptosis by NF-κB inhibition (30). In the WCE-treated animals, the prostate epithelium was improved compared to the BPH group, and cell proliferation decreased, thereby causing the decreased PI.
Another result of our study was decreased epithelial proliferation in the WCE-treated animals, characterized by the immunohistochemical detection of PCNA. PCNA is a marker for cell proliferation, and plays an important role in certain physiologic and pathologic processes. It is believed that oxidative stress is mediated by the mechanisms that are associated with prostate proliferation (31).
In the present study, induction of BPH with testosterone caused proliferation in the prostate gland, which was significantly reversed in a dose-dependent manner with WCE treatment. Kyprianou et al. have shown that downregulation of apoptotic factors, as well as increased levels of anti-apoptotic factors, decrease the rate of prostatic cell death, resulting in hyperproliferation of prostate tissue (32). Stan et al. reported that WA causes inhibition of cellular proliferation and increases apoptosis in human breast cancer cells (33). Srinivasan et al. showed that WA, by inducing the proapoptotic protein in the prostate apoptosis response-4 (Par-4), caused the arrest of prostate cancer cell growth (13). Several studies have indicated that WA can stop the G2/M cell cycle and decrease the expression of NF-kβ and TNF-α, and thus it has antiproliferative activity and induces apoptosis in cancerous cells (34, 35). It was found that WA also increased the activation of p21 in prostate cancer cells (36). Through the activation of caspases and the induction of apoptosis, p21 causes inhibition of PCNA-dependent DNA polymerase activity (37). In the present study, the immunohistochemical detection of PCNA was markedly increased in the BPH group, while it was decreased in the groups treated with WCE 500 mg/kg and 1000 mg/kg.
In conclusion, our study indicates for the first time that treatment with W. coagulans extract, in a dose-dependent manner, increases TAC levels and reduces MDA levels and proliferation in BPH. We suggest that WCE decreases the oxidative injury to the prostate cells and prevents abnormal cell proliferation, which establishes the balance between proliferation and apoptosis in BPH. This might be useful in the treatment of BPH, although more research is required in order to elucidate the potential therapeutic role of WCE against BPH.