1. Background
Chronic kidney disease (CKD) is defined as the slow and steady damage of kidney function in an irreversible manner, which ultimately results in end-stage renal disease (ESRD) (1, 2). This chronic disorder is a serious health problem with a high prevalence rate in adults and children. It causes mortality and other health complications, and high costs are incurred due to the frequent medical diagnosis and poor prognosis of patients (1, 2). The causes of CKD are very different in children than in adults. In a recent North American pediatric renal transplant cooperative study (NAPRTCS), congenital causes, including congenital anomalies of the kidney and urinary tract (CAKUT) (48%) and hereditary nephropathies (10%), were the most common causes of CKD in the children (3). Based on previous studies, such a chronic disorder impairs the quality of life of children due to the development of various clinical symptoms, especially developmental disorders and psychiatric disorders (4-6). CKD clinical manifestations in children are presented as edema, hypertension, hematuria, and proteinuria, and specifically during the neonatal period as weight gain, polyuric dehydration, and urinary tract infection (7). In addition to the above mentioned symptoms, CKD may lead to a type of hyperactivity and central nervous system (CNS) dysfunction (especially affecting sympathetic function) (8).
Based on the literature, the prevalence of cognitive disorders such as memory disorder, and different psychiatric disorders including anxiety disorders (ADs), depression, and adjustment disorders in children with different levels of CKD was significantly higher compared to the group of healthy children and children with very early stages of CKD (9-12). Based on the available literature, patients with CKD who develop psychiatric and cognitive disorders face longer hospitalization times, more health complications, and greater mortality compared to other patients at the same stage without these disorders (11, 12). Therefore, proper diagnosis of psychiatric and cognitive disorders in these patients is paramount. More specifically, one disorder whose relationship with CKD in children has not been adequately examined is obsessive-compulsive disorder (OCD).
Obsessive-compulsive disorder (OCD) is a chronic disabling illness characterized by repetitive ritualistic behaviors over which the patients have little or no control (13, 14). A review of the literature revealed no study that assessed the association between OCD (based on the obsessive compulsive inventory-child version (OCI-CV)) and CKD.
2. Objectives
The aim of this study was to investigate OCD in children with early stages of CKD and to compare it with healthy children.
3. Patients and Methods
This case-control study was performed on 160 children in the age bracket of 7 to 17 years old who were referred to the pediatric clinic of Amir Kabir hospital in Arak (Iran) in 2015. The ethics committee approved the study (approval code: 93-162-1, registration code: 1087).
Eighty children with early stages of CKD (Stages 1, 2 and 3) comprised the case group, and the control group consisted of 80 healthy children without CKD; children were included in the study based on the inclusion criteria. The sample number was calculated with regards to the prevalence of cognitive disorders due to CKD (α = 0.05%, β = 0.2%).
CKD was defined as the presence of kidney damage (for example, any structural or functional abnormality involving pathological, laboratory, or imaging findings) for ≥ 3 months or a glomerular filtration rate (GFR) < 60 mL/minute/1.73 m2 for ≥ 3 months (2). In this study, as per the CKD definition (2), children with early stages of CKD (Stage 1, 2 and 3 CKD) who were diagnosed with CKD due to renal and urinary-genital tract anomalies, such as obstructive uropathy, renal dysplasia, or reflux nephropathy, were included in the case group. Those children who were diagnosed with CKD due to reasons other than renal and urinary-genital tract anomalies, or those with stages 4 and 5 CKD were excluded from the study. Stage 1, 2, 3 and 4 CKD were defined as kidney damage with normal or increased GFR (GFR ≥ 90 cc/minute/1.73 m2), kidney damage with mild decreased GFR (GFR = 60 - 89 cc/minute/1.73 m2), kidney damage with moderately decreased GFR (GFR = 30 - 59 cc/minute/1.73 m2) and kidney damage with severely decreased GFR (GFR = 15 - 29 cc/minute/1.73 m2), respectively (15). ESRD or Stage 5 CKD was defined by the amounts of GFR < 15 cc/minute/1.73 m2 which are indicative of the start of dialysis (15-17).
We included children of both sexes in the age range of 7 to 17 years old, and children with stage 1 to 3 CKD (for at least 6 months). Written consent from patients’ parents or guardians was obtained. We excluded patients with the following conditions or circumstances: a history of considerable psychiatric disorders; intellectual disabilities, or nervous system disorders; a history of any type of anxiety disorder before developing CKD; congenital and chromosomal abnormalities; a chronic medical condition; a family history of major psychiatric disorders in first-degree relatives; parents not consenting to participating in the study; and not completing the questionnaire.
Intellectual disability was defined in terms of the intelligence quotient (IQ) of ≤ 70 (18). Healthy children were selected from children who had been referred to the hospital as outpatients for minor conditions such as the common cold or abdominal pain. The matching method was used for selecting the healthy children, and children were matched in groups based on age, sex, and socioeconomic status.
In this 3-month study (April 2015 to July 2015), a total of 40 children were excluded based on the inclusion and exclusion criteria. Among 22 (100%) patients who were excluded in the case group, 17 (77.27%) and 5 (22.72%) patients were excluded due to parental unwillingness to complete the OCI-CV and a history of considerable psychiatric disorders (anxiety disorders before the diagnosis of CKD), respectively. For the control group, 80 out of 98 investigated children were selected. The remaining children were excluded due to lack of parental consent.
After obtaining informed consent from the children’s parents, demographic, clinical, and perinatal data (age, sex, residence, birth weight, current weight, height, body mass index (BMI), mother’s age at birth, gestational age, maternal education, household incomes, marital status, type of delivery, age at diagnosis of CKD, and duration of CKD) were recorded.
OCD in children was evaluated using the obsessive compulsive inventory-child version (OCI-CV) by a psychologist (consultant). This self-reporting questionnaire has been designed for people aged 7 to 17 years, containing 21 items and 6 subscales, including doubting/checking (5 phrase), obsessing (4 phrase), hoarding (3 phrase), washing (3 phrase), ordering (3 phrase), and neutralizing (3 phrase) (19-21). The subjects are supposed to indicate their degree of agreement or disagreement with each item through a 3-point Likert scale ranging from never to always. The scoring options on this test were as follows: never = 0, sometimes = 1, and always = 2. Based on multiple sources of evidence, the OCI-CV is considered to be a reliable and valid method for identifying children with OCD. The OCI-CV was modestly correlated with obsessive–compulsive symptom severity on the children’s Yale-Brown obsessive compulsive scale (CY-BOCS), as well as with clinician-reported OCD severity (19, 20). The Persian version of the OCI-CV questionnaire was tested for reliability in a pilot study by the researchers with 30 patients in each of the case and control groups; the Cronbach’s alpha was 0.89.
The collected data was analyzed with SPSS software (statistical package for the social sciences, version 18.0, SPSS Inc. Chicago, USA) and descriptive statistical methods for frequency determination. Numerical data are expressed as mean ± SD and compared with a t test. Categorical data are expressed as numbers (percentage) and compared with a chi-square test. P values less than .05 were considered significant.
4. Results
The mean age of the children in both groups was 9.4 ± 1.7 years. The mean weight, height, and BMI of the children were 38.15 ± 6.8 kg, 127.61 ± 8.5 cm, and 17.31 ± 3.52, respectively. Of the 160 subjects, 86 (53.75%) were boys and 74 (46.25%) were girls. In the group comprised of children with CKD, the duration of the disease and the age of diagnosis were 1.48 ± 2.12 and 6.9 ± 5.4 years, respectively. The mean GFR in the case group was 59.7 ± 20.5. Table 1 shows the clinical and demographic characteristics of the children.
| Characteristics | CKD Children | Healthy Children | P Value c |
|---|---|---|---|
| Age, y | 9.5 ± 1.91 | 9.3 ± 1.4 | .211 |
| Body weight, kg | 39.1 ± 3.76 | 38.2 ± 9.23 | .51 |
| Height, cm | 126.12 ± 6.14 | 129.1 ± 6.21 | .113 |
| BMI | 17.5 ± 2.7 | 17.12 ± 2.4 | .32 |
| Gender | .112 | ||
| Female | 39 (48.75) | 35 (43.75) | |
| Male | 41 (51.25) | 45 (56.25) | |
| Birth weight, g | 2788.26 ± 614.91 | 2943.94 ± 614.10 | .08 |
| Residence | .3 | ||
| City | 68 (85) | 63 (78.75) | |
| Rural | 12 (15) | 17 (21.25) | |
| Gestational age | .12 | ||
| Full-term | 57 (71.25) | 61 (76.25) | |
| Premature (< 37 w) | 21 (26.25) | 19 (23.75) | |
| Post-term (> 40 w) | 2 (2.5) | 1 (1.25) | |
| Maternal education | .001 | ||
| College | 30 (37.5) | 16 (20) | |
| High school | 37 (46.25) | 50 (62.5) | |
| Elementary school | 13 (16.25) | 14 (17.5) | |
| Mother's age at birth, y | 25.37 ± 5.71 | 25.02 ± 5.55 | .66 |
| Household incomes d | .376 | ||
| Low | 19 (23.75) | 20 (25) | |
| Middle | 51 (63.75) | 48 (60) | |
| High | 10 (12.5) | 12 (15) | |
| Marital status | .311 | ||
| Intact marriage | 71 (88.75) | 68 (85) | |
| Separated | 9 (11.25) | 12 (15) | |
| Type of delivery | .456 | ||
| NVD | 61 (76.25) | 59 (73.75) | |
| CS | 19 (23.75) | 21 (26.25) |
aValues are presented as mean ± SD or No. (%).
bNumber of subjects = 80.
cP values less than 0.05 were considered significant.
dHousehold incomes: low mean monthly incomes < 5000000 Rials; moderate mean incomes between 5,000,000 and 10,000,000 Rials; high means incomes > 10,000,000 Rials.
The mean age (P = 0.211), weight (P = 0.51), height (P = 0.113), gender distribution (P = 0.112), average BMI (0.32), mother’s age at childbirth (P = 0.66), and the child’s birth weight (P = 0.08) were not significantly different between the children in the case and control groups. Furthermore, the children in both groups were identically distributed in terms of residency status (P = 0.3), family’s monthly income (P = 0.376), gestational age (P = 0.12), marital status (P = 0.311) and type of delivery (P = 456). However, the maternal educational level was significantly different between the two groups (P = 0.001), so that 30 (37.5%) and 37 (46.25%) of mothers in the group of the children with CKD had college and high school education, respectively, whereas 16 (20%) and 50 (62.5%) of the mothers in the control group had college and high school education, respectively.
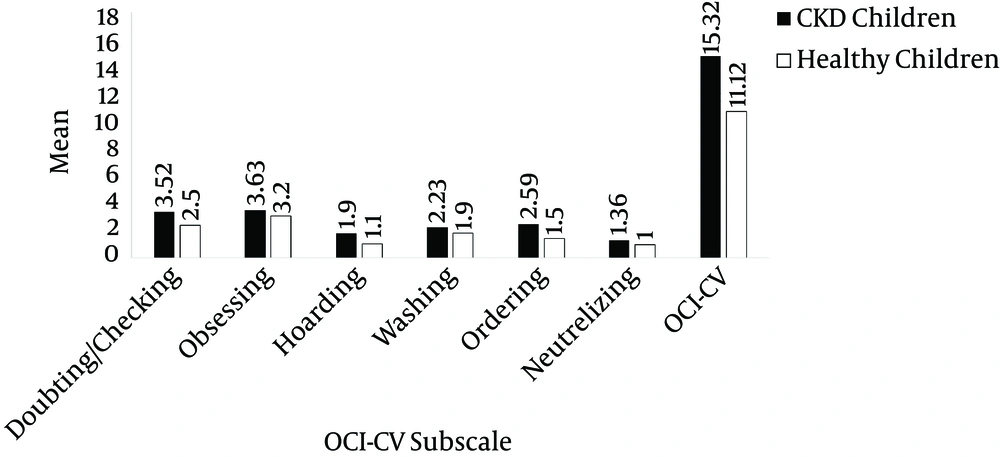
The mean scores of doubting/checking (case: 3.52 ± 2.54, control: 2.5 ± 2.32, P = 0.007) and ordering (case: 2.59 ± 1.81, control: 1.5 ± 2.56, P = 0.005) of the children with CKD was significantly higher than the scores of the healthy ones. Moreover, the mean total scores for the OCI-CV of the children with CKD at 15.32 ± 7.69 was significantly higher than those of the healthy ones at 11.12 ± 2.54 (P = 0.021). Nevertheless, the mean scores of obsessing (P = 0.11), hoarding (P = 0.117), washing (P = 0.211), and neutralizing (P = 0.41) were not significantly different between the case and control groups (Table 2 and Figure 1).
| Subscale | CKD Children | Healthy Children | P Value |
|---|---|---|---|
| Doubting/checking | 3.52 ± 2.54 | 2.5 ± 2.32 | .007 |
| Obsessing | 3.63 ± 2.48 | 3.2 ± 1.38 | .11 |
| Hoarding | 1.9 ± 1.77 | 1.1 ± 1.8 | .117 |
| Washing | 2.33 ± 2.15 | 1.9 ± 2.14 | .211 |
| Ordering | 2.59 ± 1.86 | 1.5 ± 2.56 | .005 |
| Neutralizing | 1.36 ± 1.3 | 1 ± 2.2 | .41 |
| OCI-CV | 15.32 ± 7.69 | 11.12 ± 2.54 | .021 |
aValues are presented as mean ± SD.
bNumber of subjects = 80.
According to the results of Spearman’s test, there was significant correlation between the duration of CKD and doubting/checking (P = 0.004, correlation coefficient (CC): 0.4), obsessing (P = 0.06, CC: 0.02), washing (P = 0.031, CC: 0.8), ordering (P = 0.001, CC: 0.2), and the total scores of the OCI-CV questionnaire (P = 0.04, CC: 0.4) (Table 3).
| Subscale | Correlation Coefficient (CC) | P Value |
|---|---|---|
| Doubting/checking | .4 | .004 |
| Obsessing | .02 | .06 |
| Hoarding | .12 | .131 |
| Washing | .8 | .031 |
| Ordering | .2 | .001 |
| Neutralizing | .11 | .081 |
| OCI-CV | .4 | .04 |
5. Discussion
According to the results of the current study, OCD and certain subscales of this disorder are more likely to occur in children with early stages of CKD than in healthy children. In this respect, doubting/checking and ordering had higher rates among children with CKD than among healthy children. Furthermore, the results indicated that the duration of CKD was significantly correlated with the mean total scores of the OCI-CV and certain subscales, such as doubting/checking, obsessing, washing, and ordering.
On the basis of previous evidence, chronic disorders or diseases in adults and children can be associated with reduced quality of life and social, occupational and educational poor performance (22). In fact, it has been revealed that such an association can in particular cases have a negative effect on the clinical course of the underlying disease (22). According to relevant studies, the risk of mood and anxiety disorders occurring among people with chronic medical illnesses tends to be greater as compared to the corresponding occurrence rates among healthy people in the general population (22). However, very few studies have focused on specific types of chronic diseases.
Although there have been a number of studies assessing the psychiatric disorders in children with CKD, no study has been carried out on the relationship between OCD and CKD based on the OCI-CV. Bakr et al. (9) studied 19 children with CKD who did not need dialysis and 19 others with ESRD in order to determine if any of them had a psychiatric disorder. The evidence indicated that 18.4%, 10.3%, 7.7%, 5.1%, and 2.6% of the subjects had adjustment disorder, depression, cognitive disorder, anxiety, and elimination disorder. The total prevalence of the above disorders were calculated at 68.4% and 36.8% in dialysis and non-dialysis groups, respectively. In another study on 30 children with both CKD and continuous peritoneal dialysis (CPD), 30 children who had undergone renal implantation, and 33 healthy children, Fukunishi and Honda (10) revealed that there was a significant difference in the prevalence of adjustment disorder between the children in the three groups; the dialysis and control groups had the highest and lowest prevalence of this disorder, respectively.
With the objective of investigating cognitive function in children with CKD, Slickers et al. studied 29 children aged 7 to 19 simulated through creatinine clearance (CrCl). Their IQ levels, memory, and attention levels were examined. The results showed that the severity of CKD was significantly correlated with decreased IQ and impaired memory function. Furthermore, it was revealed that a longer duration of CKD and its onset at a younger age were considerable risk factors for the development of cognitive disorders (11). In another study on children with ESRD and healthy children, Fukunishi and Kudo (23) concluded that the prevalence of anxiety and depression was significantly higher in children with ESRD and peritoneal dialysis (PD) than in healthy children. nI yet another study, Eisenhauer et al. (24) examined 15 children aged 8 to 16 with renal disease (8 children with ESRD and 7 children with mild kidney disease as the control group) and showed that the depression rate in the case group was significantly higher than that in the control group.
In 2015, Yousefichaijan et al. (25) compared the occurrence of attention deficit/hyperactivity disorder (ADHD) among 75 children aged 5 - 16 with CKD (stages 1 to 3) and 75 healthy children. The results of this study indicated that the prevalence of ADHD (case group: 12%, control group: 16%, P = 0.664) was not significantly different between the two groups. In another study on ADHD, Yousefichaijan et al. (26) examined 100 children with ESRD undergoing peritoneal dialysis and 100 healthy children. They found that the rates of attention deficit (P = 0.01) and hyperactivity (P = 0.002) among children with ESRD were significantly higher than the corresponding rates among healthy children.
According to previous studies, it can be argued that the risk of various psychiatric disorders in children with CKD at early stages and children with ESRD tend to be significantly higher as compared to healthy children. Moreover, such disorders can be found more frequently in children with ESRD, particularly in those undergoing dialysis, than in patients experiencing the early stages of CKD. Similarly, the results of this study were indicative of the higher prevalence of OCD as an anxiety disorder in children with CKD as compared to healthy children. Although it can be concluded that psychiatric interventions, particularly in the case of anxiety disorders, can be beneficial for children with varying degrees of CKD, there is an insufficient number of studies exclusively focused on each of the psychiatric disorders (such as OCD in our study) and a lack of evidence concerning the clinical impact of these disorders on the clinical course of CKD. Therefore, it is highly recommended that future studies be conducted so as to yield more definitive conclusions.
One limitation of our study involved the lack of cooperation of some parents in completing the OCI-CV questionnaire. Although this criterion led to the exclusion of some otherwise eligible children, we tried to encourage the parents by describing the possible usefulness of the study and by offering to help them fill in the questionnaire.
According to our findings, the risk of OCD among children with CKD is significantly higher than the risk among healthy children. Although the results suggest that psychiatric interventions could be helpful in treating children with CKD, the limited number of studies on this particular issue suggests that further investigation into this medical condition is required so as to obtain more conclusive results.