1. Background
Hypertension (HTN) prevalence in the normal pediatric and adolescent population ranges from 3.2% to 4.7% (1-4). Childhood HTN has been linked to target-organ damage in young adults (5). HTN is often asymptomatic in both children and adolescents; when persistent and long-standing, it could be a significant risk factor for kidney damage and increased glomerular permeability (6, 7). Proteinuria and/or urinary red blood cells (RBCs) are important urinalysis manifestations of kidney damage and increased glomerular permeability. Such evidence of bilateral kidney damage is an indication of chronic kidney disease if protracted for ≥ three months and may progress to end-stage renal disease (ESRD) if not recognized and treated early (6). Renal replacement therapy, the standard treatment for ESRD, is rarely available and affordable for many patients in developing countries (8-11). It was postulated that hypertension-related kidney damage is caused by glomerular ischemia and hypoperfusion secondary to progressive narrowing of preglomerular arteries and arterioles (12). Animal models of HTN showed that glomerular capillary HTN and hyperperfusion are the principal factors causing glomerular damage and progressive loss of kidney function (13). As a compensatory mechanism, direct transmission of high systemic blood pressure (BP) to the glomerular capillary network is physiologically blocked by an increase in afferent arteriolar resistance (14). The failure of this mechanism leads to increased glomerular capillary pressure with resultant high glomerular filtration rate, increased transglomerular passage of proteins, and mesangial influx of proteins and other macromolecules. The ultimate renal histopathology is glomerular sclerosis secondary to the activation of mesangial cells and upregulation of proinflammatory cytokines and growth factors (14).
2. Objectives
This study prospectively determined the burden of hypertension and its impact on glomerular permeability in randomly recruited primary school children.
3. Patients and Methods
3.1. Study Area and Population
This study was conducted between June 3 and November 15, 2013, among primary-school pupils, in Ile-Ife, State of Osun, Nigeria. The study location is semiurban and has two local government areas (LGAs), namely Ife central (population, n = 167,254) and Ife East (population, n = 188,087). These LGAs cover land-mass areas of 111 km2 and 172 km2, respectively.
3.2. Ethics
The study was approved by the ethics and research committee of our institution and the local inspectorates of education in the two LGAs where it was carried out. Informed consent was obtained from the parent(s)/guardian(s) of each pupil. The study conformed to the Helsinki declaration of 1975 on research involving human subjects, as revised in 2000.
3.3. Sampling Technique and Research Protocol
A multistage random sampling technique was employed to select 12 schools from a list of schools in both LGAs. Six primary schools, consisting of three private and three government-owned schools, were selected from each LGA. The school register was consulted for enrollment figures by age and gender in each of the selected schools. A composite register was drawn for subjects aged 6 - 14 years whose parent(s)/guardian(s) had consented. The subjects were categorized according to their classes; a table of random numbers was used to select the pupils. Overall, a total of 1,335 pupils were recruited and studied. The research protocol was administered on each pupil for biodata and anthropometric and clinical information. A general physical examination was carried out on each pupil before the midday break (1100 hours) in a classroom.
3.4. Blood Pressure Measurement
Following the recommendation of the task force on high BP in children and adolescents (7), resting BP was determined by the auscultation method in the right arm after a 10-minute resting period using the mercury gravity sphygmomanometer with bladder cuff sizes 17.0 - 19.0 cm (length) by 7.5 - 9.0 cm (width). Onset of the first tapping sound (Korotkoff sound 1) indicated the systolic BP (SBP), whereas the point of complete disappearance of the sound (Korotkoff sound 5) indicated the diastolic BP (DBP). For each pupil, the average of two measurements was taken after an initial BP trial run to allay fear and anxiety. Pupils with high BP had their BPs checked monthly for three months in their schools; those with persistently elevated BP were thereafter referred to our pediatric nephrology clinic for further assessment and treatment. HTN was defined as SBP and/or DBP ≥ 95th percentile for age, gender, and height on three different occasions (7). SBP and/or DBP ≥ 95th but not > 99th percentile by >5 mmHg was regarded as stage I HTN, whereas stage II HTN was defined as SBP and/or DBP > 99th percentile by more than 5 mmHg for age, gender, and height (7). BP percentile charts based on age, gender, and height developed by the task force on the diagnosis, evaluation, and treatment of high BP in children and adolescents (7) was used to determine whether a pupil was hypertensive; the same chart was used for staging of hypertension.
3.5. Anthropometric Measurement
Weight was measured using the Seca weighing machine (Seca gmbh & Co.kg, Germany). The scale was standardized daily and measured to the nearest 0.5 kg. Each child was weighed barefoot in light clothing. Height was measured with a Leicester height stadiometer (Marsden weighing machine group, UK) mounted on a vertical wall with the pupil barefoot and with head held in the Frankfort plane. The height was taken to the nearest 0.1 cm. Body mass index (BMI) was calculated as weight divided by the height squared (kg/m2). The International Obesity Task Force cutoff points of > 25 kg/m2 and > 30 kg/m2 for overweight and obesity, respectively, were used (15), whereas underweight and normal weight were defined using the BMI-for-age profiles developed by the National Center for Health Statistics for boys and girls aged 2 - 20 years (16).
3.6. Urinalysis
The presence of protein and/or red blood cells (RBCs) in the urine was assessed using the UriScreen Combi 10 dipstick (Yercon Diagnostic Co., Ltd., Chang Chu, China). Freshly voided urine samples were collected into plane universal bottles prior to the pupils’ midday break. The test strip was dipped into the fresh urine for approximately 1 sec and then drawn across the edge of the container to remove excess urine. After 30 seconds, the test strip was compared with the color scale, and the result was immediately recorded. Color changes after 2 minutes were disregarded. Urine protein level was assessed as negative, trace, 1 + (30 mg/dL), 2 + (100 mg/dL), and 3 + (500 mg/dL). In this study, significant proteinuria by dipstick testing was defined as urinary protein ≥ 30 mg/dL (≥ 1 +). A dipstick value ≥ ca. 5 - 10 erythrocytes/µL was taken as significant hematuria. Like their BP, the pupils’ urine was examined monthly for significant proteinuria and/or RBCs for three months. According to the national kidney foundation-kidney/disease outcome quality initiative (6), persistent significant proteinuria and/or urinary RBCs determined by the dipstick test for three months or more is regarded as evidence of abnormal glomerular permeability.
3.7. Data Analysis
Statistical analysis was performed using SPSS 16 for Windows, evaluation version (2006 SPSS Inc.). The comparative statistics used were Student’s t-test, and Pearson’s correlation (r). Other data were presented as numbers and percentages. A P value < 0.05 was regarded as statistically significant.
4. Results
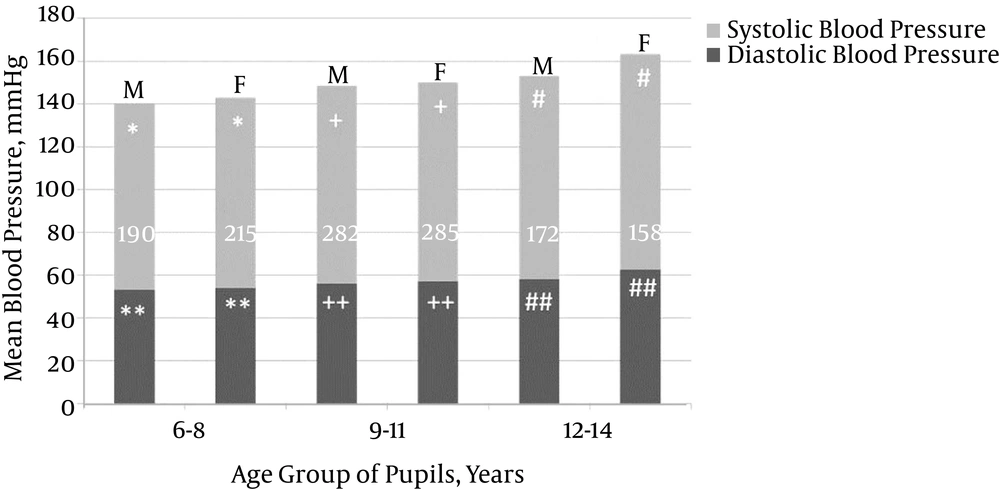
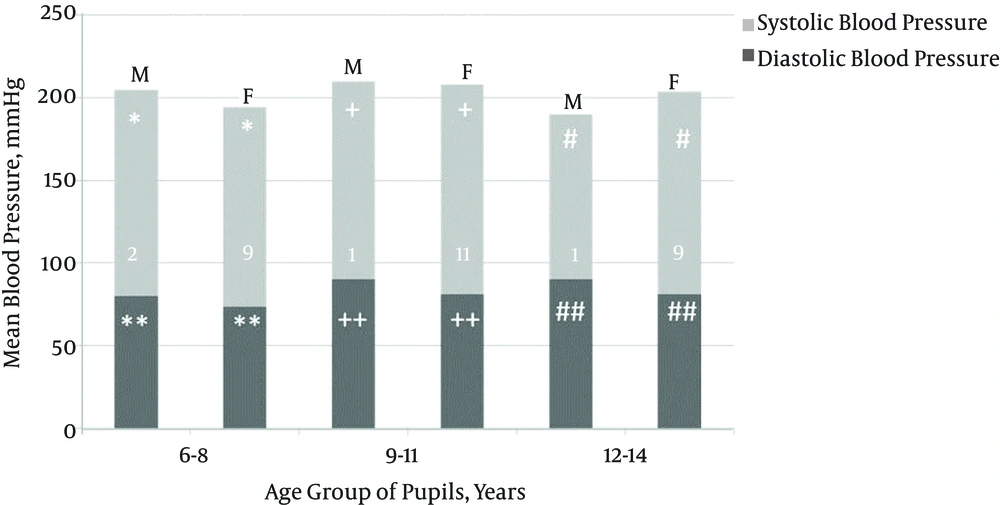
The study population included more females (n = 687; 51.5%) than males (n = 648; 48.5%). Of 1,335 (1.9%) pupils, 25 had prehypertension, and 33 (2.5%) pupils aged 6 - 14 (10.0 ± 2.4) years were hypertensive; the majority of them were females (29/33; 88.0%), resulting in a male-to-female ratio of 1:7. The mean SBP/DBP in the hypertensive pupils was 125.6 ± 6.5/81.7 ± 3.3 mmHg (range: 114.0 - 140.0/80.0 - 90.0; > 95th to > 99th/ > 95th to 99th percentile). There was a weak but significant correlation between SBP (r = + 0.334, P = 0.01), DBP (r = + 0.278, P = 0.01), and age. Gender comparisons of mean SBP and DBP by age group in normotensive and hypertensive school pupils are respectively, summarized in Figures 1 and 2. Table 1 shows the number of pupils per HTN stage by age group and gender, and Table 2 summarizes the pattern and stages of HTN in the pupils.
| Blood Pressure (BP) by Age Group | Systolic and Diastolic Hypertension Stage | Gender | Total | |
|---|---|---|---|---|
| Male | Female | |||
| 6 - 8, y | ||||
| Systolic BP, mmHg | I | 0 | 6 | 6 |
| Diastolic BP, mmHg | I | 1 | 1 | 2 |
| Systolic BP, mmHg | II | 1 | 2 | 3 |
| Diastolic BP, mmHg | II | 0 | 0 | 0 |
| 9 - 11, y | ||||
| Systolic BP, mmHg | I | 0 | 4 | 4 |
| Diastolic BP,mmHg | I | 0 | 0 | 0 |
| Systolic BP, mmHg | II | 0 | 5 | 5 |
| Diastolic BP, mmHg | II | 1 | 2 | 3 |
| 12 - 14, y | ||||
| Systolic BP, mmHg | I | 0 | 3 | 3 |
| Diastolic BP, mmHg | I | 1 | 4 | 5 |
| Systolic BP, mmHg | II | 0 | 2 | 2 |
| Diastolic BP, mmHg | II | 0 | 0 | 0 |
| Type and Stage of Hypertension | No. (%) | Mean Blood Pressure (BP), Mmhg (Range) | BP Percentile |
|---|---|---|---|
| Isolated systolic hypertension | 14 (42.4) | 125.0 ± 6.7 (114.0 - 140.0) | > 95th to 99th |
| Isolated diastolic hypertension | 10 (30.3) | 82.0 ± 3.5 (80.0 - 90.0) | 95th to 99th |
| Combined systolic and diastolic hypertension | 9 (27.3) | 126.7 ± 5.7/80.0 ± 0.0 (120.0 - 130.0/80.0–80.0) | > 95th to > 99th/99th |
| Stage I hypertension | 20 (60) | ||
| Systolic BP | 121.0 ± 4.5 (114.0 - 130.0) | 95th to 99th | |
| Diastolic BP | 80.5 ± 1.2 (80.0 - 84.0) | 95th to < 99th | |
| Stage II hypertension | 13 (40) | ||
| Systolic BP | 131.0 ± 3.3 (130.0 - 140.0) | 99th to > 99th | |
| Diastolic BP | 86.5 ± 4.4 (80.0 - 110.0) | 99th to > 99th |
The overall prevalence of overweight and obesity among the 1,335 pupils was 0.4% and 1.4%, respectively, but none of the hypertensive pupils was obese. HTN was, however, detected in 25 (75.8%) normal weight and seven (21.2%) underweight pupils and in one (3.0%) overweight pupil. The mean heights for the hypertensive male and female subjects were 132.4 ± 6.91 cm and 138.80 ± 10.44 cm, respectively (P = 0.04), and the mean BMIs were 16.12 ± 2.11 kg/m2 and 15.91 ± 1.76 kg/m2, respectively (P = 0.72). Overall, SBP (r = +0.213; P = 0.01) and DBP (r = +0.148; P = 0.01) correlated weakly with BMI. SBP (r = +0.434; P = 0.01) and DBP (r = +0.389; P = 0.01), however, correlated fairly well with height. Analyses for gender difference showed poor correlations between SBP, DBP, mean arterial pressure (MAP), and BMI as well as height (Table 3).
| Blood Pressure (BP) | Correlation Coefficient, R | |||
|---|---|---|---|---|
| Body Mass Index | Height | |||
| Male | Female | Male | Female | |
| Hypertensive pupilsa | ||||
| Systolic BP | - 0.015 | - 0.165 | + 0.366 | + 0.008 |
| Diastolic BP | - 0.035 | - 0.068 | + 0.603 | + 0.274 |
| MAP | - 0.035 | - 0.203 | + 0.673 | + 0.315 |
| Normotensive pupilsb | ||||
| Systolic BP | + 0.207 | + 0.249 | + 0.380 | + 0.496 |
| Diastolic BP | + 0.133 | + 0.178 | + 0.319 | + 0.452 |
| MAP | + 0.171 | + 0.220 | + 0.970 | + 0.503 |
aP = 0.05.
bP = 0.01.
Urinalysis by dipstick revealed no evidence of abnormal glomerular permeability, because none of the hypertensive pupils had significant proteinuria and/or urinary RBCs. Trace proteinuria, however, was found in three of the 33 (9.1%) hypertensive pupils.
5. Discussion
The 2.5% prevalence of HTN among school children in this study, determined based on the fourth task-force criterion (7), is similar to the 2.2% prevalence reported among Swiss school children by Chiolero et al. (17) but is 1.3 times lower than the 3.2% prevalence reported four years earlier by Adegoke et al. (1) in the same locality. The disparity is probably due to the higher upper-age limit of 18 (range: 6 - 18) years in the earlier study compared to 14 (range: 6 - 14) years in the current study. In both studies, HTN was diagnosed mostly for the first time in adolescent pupils, thus underlining the need for early screening of school children for hypertension, because target-organ damage in adulthood had been traced to childhood HTN (5, 18).
Isolated HTN was more frequent (72.7%) than combined systolic and diastolic HTN (27.3%) in this study. Furthermore, isolated systolic HTN (SHTN) prevalence (42.4%) was higher than isolated diastolic HTN (DHTN; 30.3%), confirming earlier reports that isolated HTN is not rare (19, 20). Studies by Rosner et al. (19) (SHTN, 4.4% vs. DHTN, 3.2%) and Sorof et al. (20) (SHTN, 47% vs. DHTN, 17%) revealed that isolated SHTN is more common than isolated DHTN. The pathophysiology of isolated HTN in children is not yet clear; however, studies have shown that isolated SHTN is more frequently linked with end-organ damage than is isolated DHTN (21, 22). As shown in Figure 1, the mean BP of the normotensive school children tended to increase with increasing age, especially as the children approached pubertal age. This is similar to findings in earlier studies (1, 4, 23-26). This pattern, which can be imputed to normal physiological hormonal changes that attend puberty and the increase of peripheral arterial resistance and cardiac output with age (27), was not replicated in the hypertensive children. In the latter, BP rise was haphazard and failed to correlate with rising age (Figure 2). This suggests that hypertension is associated with a defective normal BP regulatory mechanism.
The mean hypertension age was 10 years, and the majority of the hypertensive pupils had stage I HTN (60.0%). Whether they had stage I or II HTN, the female children tended to be more hypertensive. This may also, in part, be due to earlier puberty onset in females than in males. However, the very high prevalence of stage II HTN (40.0%) in this study indicates the possibility of a secondary etiology for hypertension in a good number of the pupils. In one study, 35% - 50% of hypertensive adolescents were obese, with a positive correlation established between obesity and HTN as early as 5 years of age. The study argued that obesity causes and sustains childhood essential hypertension (28). In another study, HTN prevalence was found to increase progressively with increasing BMI, and approximately 30% of overweight school children had HTN (BMI > 95th percentile) (29). Although these findings agree with those from Port Harcourt (18), a highly Westernized cosmopolitan city in Nigeria where the prevalence of overweight and that of obesity among school children were 5.7% and 5.9%, respectively, they are at variance with the findings from this study. The prevalence of overweight and that of obesity were 0.4% and 1.4%, respectively, but none of the hypertensive pupils was obese; BMI correlated poorly and inversely with BP in the hypertensive pupils in this study. In fact, the majority of the hypertensive pupils had either normal or low BMI. This is consistent with a US study that revealed high BP at low BMI among Black children, indicating a weaker effect of BMI on BP levels in Blacks (19). It is not clear why the majority of the hypertensive pupils in this study had either normal or low BMI. Perhaps an investigation of the etiology of HTN would have made the reasons for this obvious. Environmental factors, sleep disorders, low birth weight/reduced nephron number, positive family history of HTN/cardiovascular disease, and genetic disorders are a few of the factors that have been associated with HTN (30). Some congenital anomalies of the kidneys and urinary tract and even renal artery stenosis and endocrine disorders could have been missed in the children, because the study was not designed to establish the etiology of HTN. Although all of these may constitute limitations of this study, they point to future avenues of research on HTN in school children. Future studies should, therefore, focus not only on HTN prevalence but also on HTN etiology so that management plans can be more robust and definitive.
Evidence for abnormal glomerular permeability could not be established in this study, because none of the hypertensive pupils, including those with stage II HTN, had significant proteinuria and/or urinary RBCs. This may indicate that HTN was not long-standing at the time of the study or that the method used was not sensitive enough to detect abnormal glomerular permeability in HTN. Palatini et al. (31) showed that young adults with stage I HTN and hyperfiltration developed microalbuminuria three times more often than normally filtrating patients after eight years of follow-up, supporting the argument that the impact of increased BP on renal structures depends not only on high BP levels, but also on how long the BP has been elevated (14).
Trace proteinuria, a risk factor for ESRD (32), was found in 9.1% of the hypertensive pupils. The pupils with trace proteinuria who were regarded as normal in this study may have had microalbuminuria, but the convention of regarding trace proteinuria as negative has the tendency to exclude a large number of people with microalbuminuria (33). Trace proteinuria may serve as an important marker of microalbuminuria in both the general population and those at high risk of cardiovascular disease (33). The dipstick test of ≥ +1 that is considered positive evidence of proteinuria has been reported as an unsuitable test in the general population because of its low sensitivity for urinary abnormalities (34, 35). Trace proteinuria should therefore be an indication for exclusion of microalbuminuria in the hypertensive person. The dipstick test showing trace proteinuria may therefore be a useful tool for that purpose (36-38).
5.1. Conclusion
The burden of HTN was 2.5%, with high BP commonly found at normal BMI. Young females were more frequently hypertensive than male school children, indicating the need for routine BP checks in school-aged female children. Abnormal glomerular permeability was rare in the young hypertensive school children, suggesting either recent hypertension or an insufficiently sensitive evaluation method. Testing for microalbuminuria and a urine microscopy examination for RBCs and other urine sediments might be more informative and predictive with respect to abnormal glomerular permeability in hypertension.