1. Background
Drug abuse is rising dramatically, with catastrophic consequences (1). In addition to mental and physical dependence, drugs also affect physical parameters, with changes that may continue even after stopping the drugs. The genitourinary system can be affected by drugs, and due to its role in continuing the human race, research about the effects of drugs on this system is of extreme importance. Some studies have indicated that oral opium can decrease luteinizing hormone (LH), dihydrotestosterone, and follicle stimulating hormone (FSH), and can cause hypogonadism in 89% of abusers (2). The prevalence of erectile dysfunction and decreased libido is also significantly higher in drug abusers (3).
Recent investigations have shown that the abuse of morphine and other narcotics significantly decreases testosterone levels (4). It is also known that chronic morphine consumption affects LH, testosterone, and body weight in rats, while no considerable changes have been observed in FSH and testicular weight (5). These findings are also supported by Cicero et al.; however, they reported a decrease in testicular and seminal vesicle weight (6). The influence of morphine on hypothalamic monoamine levels has been well-established in rats (5, 7). Gabriel et al. evaluated the effect of morphine and testosterone on rats, and concluded that morphine and testosterone together suppress the gonadotropin-releasing hormone (GnRH) (7).
The aim of this study was to determine the various effects of drugs on the genital system of rats, in order to provide a basis for human experiments. We stimulated the effects of morphine on hormonal (LH, FSH, and testosterone) and histological parameters of rat testicles.
2. Objectives
The aim of this study was to determine the short-period influence of chronic drug abuse on sex hormones and spermatogenesis in rats.
3. Materials and Methods
This was an interventional study. Thirty adult male rats (60 days old) were consecutively divided into two groups of 15 rats each, using a simple randomized method. After weighing, the case group was subjected to injections of 5 mg/kg of peritoneal morphine every 12 hours with an insulin syringe. Normal saline was injected in the control group under similar conditions. To provide the same psychological conditions, both groups were injected simultaneously. According to previous studies, the injections were performed for 60 days, approximately twice the duration of spermatogenesis (6).
3.1. Sampling Procedure
After 60 days, each rat was weighed. Under general anesthesia with ether, blood samples were taken from the carotid artery and collected in dry tubes. Serum was collected and used for hormonal analysis (FSH, LH, and testosterone). The rats then underwent orchiectomy. A ventral midline incision was made to access the testes, then the epididymis was removed and the terminal parts were clamped. The kidneys, ureters, and ventral prostate were also detached. The testicles and seminal vesicles were separated and weighed for each group, and the samples were sent to a pathologist for review.
3.2. Pathological Assessment
The testicles were placed in 10% formalin and sent for pathological assessment. A questionnaire was completed for each rat. The pathological variables included seminiferous tubule diameter, sperm count per high-powered field (HPF), sperm morphology, testis weight, spermatogenesis rate, sperm motility, and Sertoli and Leydig cell indices.
Sperm motility was scored using a 5-grade scale: normal motility (grade 5), fast sperm movement in a straight direction with little deviation (grade 4), slower movement compared to grade 4 (grade 3), slower movement with great deviation (grade 2), without forward movement (grade 1), and without movement (grade 0).
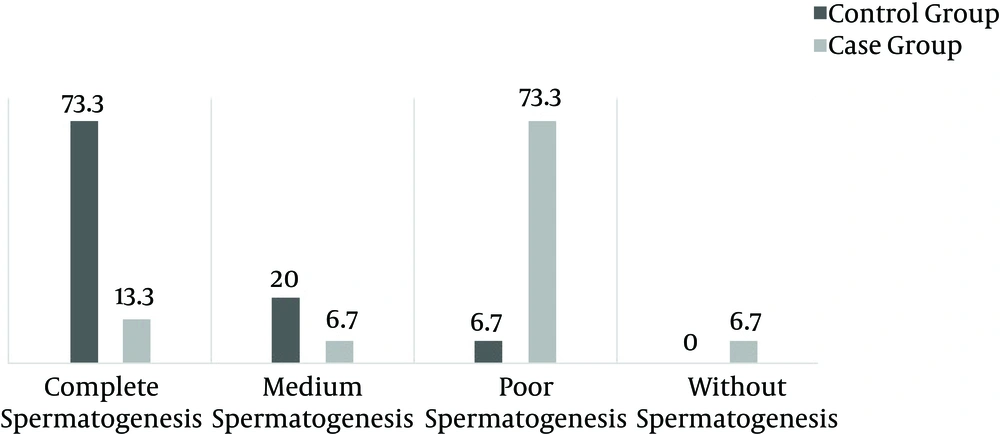
The spermatogenesis rate was determined by analyzing 10 microscopic samples and the rates were divided into groups: more than 50% complete spermatogenesis in tubules was classified as complete spermatogenesis, 25% - 50% spermatogenesis in tubules was classified as medium spermatogenesis, 1% - 25% spermatogenesis in tubules was classified as poor spermatogenesis, and < 1% spermatogenesis in tubules was considered no spermatogenesis.
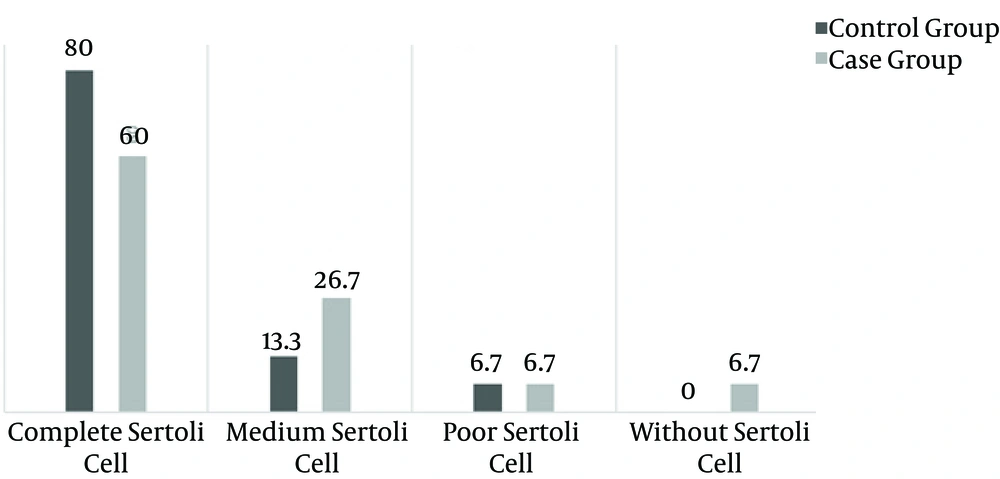
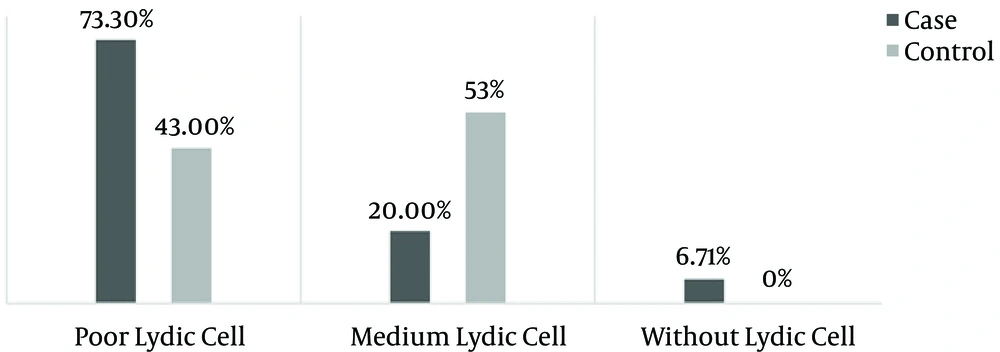
Sertoli and Leydig cell indices were determined on the basis of cell counts of each kind, in 10 microscopic samples. More than 50 cells was considered normal, 10 - 50 cells was considered normal, and 5 - 10 cells was considered low.
LH, FSH, and testosterone were measured using human kits or RIA methods. Finally, data were collected for statistical analysis.
3.3. Statistical Analysis
Data from the biochemical and histopathological findings were analyzed with SPSS 11.5. Descriptive data were explained using measures of dispersion and the frequency distribution. For comparing serum hormone levels and weight changes, t-tests were used. The Mann-Whitney test was applied for qualitative rating. Morphology was assessed using the chi-square test. P < 0.05 was considered significant.
4. Results
The mean weight of the control group was significantly higher than in the case group (P = 0.013). Mean seminiferous tubule diameters were not statistically different between the two groups (P = 0.094). Sperm counts were defined as the number of sperm per HPF. The mean sperm count was more than three times higher in the control group (P < 0.001). LH serum levels were significantly different between the two groups (P = 0.002), while FSH and testosterone serum levels showed no significant differences (P = 0.966 and P = 0.263, respectively). Also, no significant differences were observed in testis weight (P = 0.176). These results are shown in Table 1.
| Parameter | Case Group | Control Group | P Value |
|---|---|---|---|
| Mean weight, g | 134 | 157 | 0.013 |
| Mean seminiferous tubule diameter, mm | 135 | 165 | 0.094 |
| Mean serum LH level, mg/dl | 3.41 | 3.79 | 0.002 |
| Mean serum FSH level, mg/dl | 7.05 | 7.06 | 0.966 |
| Mean sperm count per HPF | 1.2 | 4.5 | 0.001 |
| Mean testosterone serum level | 2.34 | 3.1 | 0.263 |
| Mean testis weight | 3.06 | 2.87 | 0.176 |
Serum LH levels were significantly different between the case and control groups, but there were no differences in serum FSH and testosterone levels. There were no differences in mean weight or seminiferous tubule diameter between the two groups.
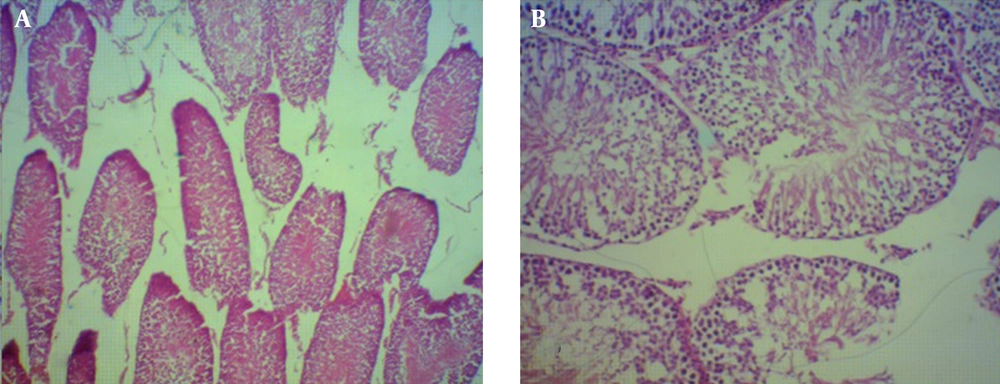
Spermatogenesis was significantly decreased in the case group (P < 0.001) (Figures 1 and 2). Sperm morphology was not significantly changed in the rats after using morphine, compared to the control group. Similarly, the Sertoli and Leydig cell indices were not significantly different between the two groups (Figures 3 and 4). Sperm motility appeared to be dramatically decreased in the case group (P < 0.001).
5. Discussion
In this study, we investigated the association between drug abuse and sexual function in an animal model, as well the possible impact of chronic morphine exposure on spermatogenesis and sexual hormone levels.
Maternal opioid use has significant effects on fetal outcomes, and offspring neurodevelopment has been an area of interest for decades, with studies of the effects of opium use by mothers on the health and the neurodevelopment of the fetus (8, 9). The use of morphine during the teenage years has also been studied by many researchers. By using morphine in male rats during adolescence, scientists have identified important changes in sexual maturation. When these males were mated with untreated females several weeks after morphine exposure, they produced smaller litters, and parameters such as LH, sex adrenal-specific changes, and hypothalamic β-endorphins have shown significant changes in the offspring of male rats that have been given morphine (6). In the present study, we observed a significant difference in LH between the morphine-induced cases and the control rats.
In contrast to our results, in other studies, female rats received morphine during early-to-mid adolescence. The morphine was discontinued for several weeks, and then these females were mated with morphine-free males. In these studies, there were no significant differences in any postnatal parameter (i.e. litter size, weight, sex ratio); however, the offspring of opium-treated rats demonstrated significant changes in behavior and emotional and sex features (10, 11).
Luteinizing hormone releasing hormone (LHRH), which is released from the hypothalamus, causes secondary effects on the pituitary gland and the release of LH and then testosterone from the testes. Our study showed that the release of testosterone was reduced in the morphine-treated group, but whether the reason for the decreased testosterone was cellular dysfunction of the testis or due to decreased LH is unknown. Also, the level of LH in the bloodstream was decreased in the case group. We did not know whether the decreased LH was due to pituitary dysfunction or to the decreased release of LHRH in the case group. It is possible that LHRH, LH, and testosterone were all affected by the morphine (6, 12, 13).
In our study, histological examinations indicated that the mean seminiferous tubule diameter and testis weight were not significantly different between the case and control groups; however, in similar studies, testis and seminiferous sizes decreased significantly. These researchers claimed that stromal atrophy of the genital organs and testosterone function in restoring the structure of seminal vesicles was the reason for the decreased size of the genital organs due to morphine (14).
The differences between the effects of morphine and oxymorphone on female fertility are not obvious. The acute doses used in morphine studies are higher and the route of administration is different than that used for oxymorphone. Despite the effects of morphine on the reproductive system and fertility of male rats, some studies showed that oxymorphone did not affect these parameters. In another study, oxymorphone was administered for 4 weeks before mating and there were no effects on the reproductive system. There were also no effects on sperm count or motility, or on the testes, seminal vesicles, and prostate weights in males after approximately 9 weeks of dosing; however, our study indicated that morphine could affect spermatogenesis and the Sertoli cell count. These results are in contrast to our study and others on the male reproductive effects of morphine (15), in which administration of morphine to male rats for 2 weeks (30 mg/kg twice daily) resulted in marked decreases in the number of matings, pregnancies, and implantations per litter, and an increase in pseudo-pregnancies when the animals were mated with untreated females. These findings were not associated with decreased sperm count or motility. However, seminal vesicle and prostate weights were significantly reduced in the morphine-treated males. The authors concluded that the effects of morphine on male fertility were likely secondary to the effects on accessory sex gland secretions and potentially on the formation of the copulatory plug (16-18).
In our study, we observed that the mean sperm count and motility, and also the rate of spermatogenesis, were significantly lower in the male rats who were administered morphine.
As with females, the basis for the differences between morphine and oxymorphone on male reproductive function is not entirely clear. In the previous study, however, the dose of morphine used (30 mg/kg administered subcutaneously twice daily, or 60 mg/kg/day) (15) was 30-fold higher than the ED50 level for analgesic activity (2 mg/kg for males by subcutaneous injection) (19). Lower doses were not evaluated in that study. In the present study, the dose of morphine used for the target group (5 mg/kg intraperitoneal injections, twice a day) was lower compared to the previous study.
5.1. Limitations
A study design that determines the influence of morphine on reducing the exact hormones (LH, FSH, or testosterone) is essential, and discovery of the mechanism of morphine’s effects on the genital and hormonal systems is needed in future studies. Human studies will be necessary to identify the exact effects of morphine on the human reproductive system.
5.2. Conclusion
If the results of this study could be generalized to humans, with regard to morphine’s properties and its increasing usage among human populations, infertile individuals with a history of drug abuse could be informed about the known short- and long-term effects on the genital system. Many of these effects, especially hyaline degenerative changes of the seminiferous tubules, are irreversible and result in permanent infertility.