1. Background
The presence of < 103 spermatozoa/mL in seminal fluid after centrifugation is called “cryptozoospermia” that none are seen in initial evaluation (1). Patients with cryptozoospermia also suffer from transient azoospermia, which means that only a few mature spermatozoa may be found in their ejaculate, and hence are Infertile because of virtual azoospermia (2). This is likely due to floating spermatogenesis in some severe non-obstructive azoospermia, thus usually the detection of sperm on the day of oocyte retrieval is unpredictable (2). Moreover if specimens yield no sperm cells after repeated ejaculations in cryptozoospermia, the clinicians can consider the testicular sperm extraction (TESE) or epididymal sperm extraction procedures as defined in the World Health Organization manual for cryptozoospermia (3). to prevent ICSI procedure canceling in transient azoospermic patients, sperm cryopreservation is also recommended (4). The cryptozoospermia may be the result of hypogonadism or sporadic spermatogenesis and may be seen in genetic anomalies, such as microdeletion of Y chromosome in AZF region (5).
Testicular or epididymal sperm retrieval procedures are available options when the outcome of fertilization by ejaculated sperms is not favorable or when no clinical pregnancy is achieved after multiple attempts with ejaculated fresh sperm cells (6). When both sources of spermatozoa are available (i.e. ejaculated or testicular), there is currently no standard of care for the choice of spermatozoa for the ICSI. A few studies have compared fertility outcome between ejaculated sperm and fresh testicular/epididymal sperm cells. Weissman et al. have reported better pregnancy outcomes with fresh testicular sperm cells in four cases which had frequently failed in vitro fertilization (IVF) / ICSI cycles with ejaculated sperms (7, 8). This is confirmed by Bendikson et al. results that compared 16 cases of fresh testicular or ejaculated sperm and reported a trend in favor of testicular sperm (9). On the contrary, a case report from Koscinski et al. demonstrated in a cohort study that ejaculated sperm was preferred to testicular sperm in ICSI procedure (10). Finally, a recent meta-analysis acknowledged that existing literature does not suggest a strong recommendation or clinical preference for testicular or freshly ejaculated sperm in the setting of idiopathic cryptozoospermia with ICSI (1).
In this prospective cohort study, we hypothesized that in CO patients, ICSI with surgically retrieved sperm (versus freshly ejaculated sperm) can lead to higher fecundability.
2. Objectives
We aimed to determine the most appropriate treatment option for patients with idiopathic cryptozoospermia considering the predictors of fertility and embryo quality.
3. Methods
This was a prospective cohort study of 152 ICSI cycles performed between May 2011 and January 2014 on 107 cryptozoospermic male partners. We obtained institutional review board (IRB) approval as well as individual patient signed consents. After excluding 24 patients (because of incomplete follow-up and lack of reliable information), the treatment cycles were divided randomly (1:1 in order of referring) into two main groups for ICSI: 1) group with a freshly ejaculated sperm (group 1; n = 42), and 2) group with sperm obtained from testicles/epididymis (group 2; n = 41).
Sperm retrieval in group 1: sperm was obtained in group 1 by ejaculation 3-6 days after abstinence. The sample was kept at 37°C for up to 30 minutes or when it liquefied completely. We performed sperm washing procedure by using discontinuous two-layer colloidal density gradient centrifugation. The ejaculate and the gradients were centrifuged at 300 G for 25 minutes. The supernatant was discharged and the bottom layer was diluted with HydroxyEthyl PiperazineEthaneSulfonic acid (HEPES), a buffered medium, and washed again by centrifugation for 10 minutes. The pellet was suspended in 200 microliters of a HEPES buffered medium and maintained at 37°C until the time of ICSI. The mean repeat of sample in this group was 3.5 times. In four patients, however, we failed to obtain ejaculated sperm; thus, they were excluded.
Sperm retrieval in group 2: Sperm was retrieved from the testis or epididymis under local anesthesia. First, the percutaneous epididymal sperm aspiration (PESA) was performed with a 26-G needle attached to a tuberculin syringe containing about 0.1 mL of sperm-washing compound. If we were unable to extract suitable sperm, we proceeded with microsurgical-guided open biopsy. The dissection of seminiferous tubules was performed mechanically under stereo-microscopy in HEPES-buffered medium. The supernatant was collected for sperm assessment. Sperm evaluation was conducted at 400 × magnification using a phase-contrast inverted microscope. In this group, six patients were excluded for fear of operation and anesthesia. Also, in this group for the selection of viable sperm for intracytoplasmic sperm injection, we used modified hypo-osmotic swelling test (single sperm curling test) (11).
IVF hyperstimulation protocols were performed using modified superlong protocol and 1.875 mg GnRH agonist was intramuscularly injected twice in mid-luteal phase and then, human menopausal gonadotropin (HMG) was started 16 days after the second GnRH agonist injection and oocytes were fertilized in standard ICSI procedure. Embryos were transferred on day 2 or 3 (the eight-cell stage).
We have defined the fertilization rate as the number of normally fertilized ovums divided by the number of ovums injected or inseminated. Implantation rate in each pregnancy cycle was determined by dividing the sacs seen on the initial ultrasound by the total number of embryos transferred during the treatment cycle resulting in pregnancy.
The outcome was defined as clinical pregnancy from which, there were 3 spontaneous abortions (5 - 20 weeks of gestation) in group 1 and 3 ectopic pregnancies (1 in group 1 and 2 in group 2). Embryonic morphologic assessment was performed at two times by the inverted microscope first on day 2 after the ovum pick-up and then on day 3. The embryos at the eight-cell stage with less than 20% fragmentation were described as good quality embryos.
In this study as mentioned above, the primary outcome was clinical pregnancy that was confirmed by a gestational sac and fetal heart beat on transvaginal ultrasound at 6 - 7 weeks of pregnancy and a previous implantation rate as defined earlier. Secondary outcomes were fertilization rate and cleavage rate. The former was defined as the number of fertilized eggs out of total eggs that inseminated or injected.
Data were expressed as mean ± SD and analyzed using SPSS version 18.0. Student’s t-test was used to compare continuous variables between the groups, and X2 and Fisher’s exact tests were used for categorical variables. In all cases, statistical significance was set at P < 0.05.
4. Results
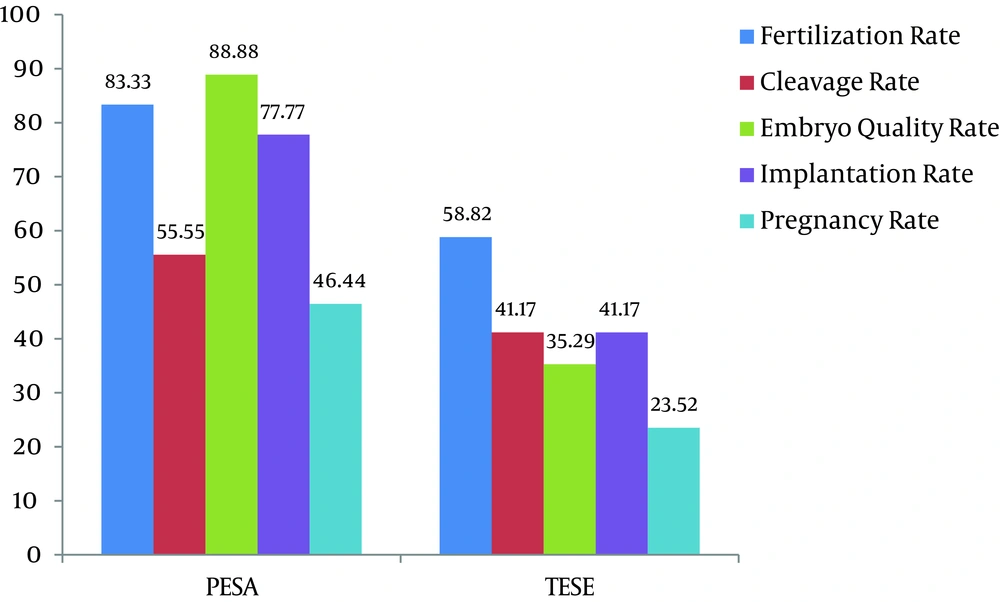
Seventy-three men with idiopathic cryptozoospermia (< 103 spermatozoa/ml) were randomly allocated to two groups in a 1:1 fashion. Group one (n = 38) used freshly ejaculated sperm and group two (n = 35) had sperm obtained from extraction via epididymis (n = 18) or testis (n = 17). All patients in both groups had idiopathic oligoasthenospermia and there was no significant difference in age, duration of infertility, and type of infertility (Table 1). Table 1 demonstrates the comparison of pregnancy outcomes between two groups. There were significant differences in fertilization rate, embryo quality rate, implantation rate, and pregnancy rate between the groups. In group 2, 18 patients (51.42%) with PSEA and 17 (48.57%) with TESE were also significantly different in fertility outcomes (P > 0.05). The PESA sub-group outcomes were significantly better than those of TESE, particularly in terms of pregnancy rate and embryo quality rate (P < 0.05) (Figure 1).
| Variables | Group 1 (n = 38) | Group 2 (n = 35) | x2 | df | P |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Implantation rate (%) | 39.47 | 77.14 | 37.97 | 1 | < 001 |
| Clinical pregnancy rate (%) | 31.57 | 57.14 | 25.54 | 1 | < 001 |
| Secondary outcome: | |||||
| Fertilization rate (%) | 55.26 | 85.71 | 32.52 | 1 | < 001 |
| Cleavage rate (%) | 57.14 | 66.66 | 10.62 | 1 | > 0.05a |
| Embryo quality rate (%) | 39.47 | 77.14 | 21.45 | 1 | < 05 |
anon-significant.
5. Discussion
ICSI is indicated in the treatment of male infertility due to severe oligospermia and cryptozoospermia (12). Currently, the standard of care for sperm source selection remains unclear. In this study, we compared freshly ejaculated sperm pregnancy outcomes with TESE and PESA ejaculated sperm using ICSI in this population. In our study, the clinical pregnancy outcome in group 2 (PESA or TESE) was higher than that of the control group. The two highest quality embryos from both groups were transferred into the uterus on day 3 although others prefer 3 days to 5 days transfer according to embryo selection criteria (13).
With the advent of ART and ICSI in the 1990s, there were significant improvements in fertility rates (14). ICSI allowed men with severe sperm abnormalities achieve parenthood. Severe oligoasthenozoospermia, cryptozoospermia, and non-obstructive azoospermic patients can be successfully cured with ICSI (15). In cryptozoospermic cases, only very meticulous methods of sperm collection with repeated semen samples allow the retrieval of a rare sperm. In addition, the absence of or insufficient number of spermatozoa in the semen of these patients on the day of the ovum retrieval can lead to the cancellation of an ICSI procedure. These situations require sperm extraction from the epididymis or testis, or in some cases, ovum cryopreservation in the next time of successfully ejaculated sperm.
In order to avoid the cancellation of ICSI in these cases, most infertility centers provide an alternative surgically sperm extraction (PESA or TESE) (10) synchronous to oocyte retrieval (16); however, there is a debate about outcomes of ejaculated sperm and extracted sperm in literature. Therefore, we conducted a prospective cohort study to highlight outcome differences in these two methods of sperm retrieval.
Although a body of research implies that the freshly ejaculated sperms are better than surgically retrieved sperms for ICSI, we found contrary results. Abduljabbar et al. reached to this conclusion that the fertilization rates are not affected by the source of the sperm but the quality of embryos gets better with ejaculated sperm when compared with sperm from TESE which corroborated in a case report on men with cryptozoospermia (10, 17). Nagy et al. (1995) and Aboulghar et al. (1997) reported that the fertilization and pregnancy rates by testicular spermatozoa were significantly lower than those of ejaculated and epididymal spermatozoa in non-obstructive azoospermia (10, 15). The sperm extraction proportion from testicular and epididymis in our study was similar but fertility outcomes in PESA sub-group was significantly better than outcomes of TESE sub-group, similar to the results of Alrabeeah et al. (16). In contrast to our study results, Some researchers believe that the fertilization rate of testicular extracted spermatozoa from non-obstructive azoospermic men is comparable to ejaculated spermatozoa in men with unexplained fertility and also stated that the fertilization rate of severe oligospermia or cryptozoospermia is similar to that of testicular spermatozoa source (15, 18, 19).
In this study, cleavage rate (74.62% and 83.43%) as well as implantation rate (21.35% and 35.17%) were significantly higher in embryo transfer, similar to the study by Popal et al. (7) and Mangalraj et al. (9, 10).
In our study, embryo quality and hence cleavage rate between two groups were not different, but the quality of embryo in sperm-extracted group was better than that of sperm-ejaculated group (< 0.05).
We believe the cryozoospermic niche is unique. In this state, the sperm encounters in the genital tract with hazardous environment, contaminating cells, and biochemical agents (e.g. reactive oxygen species). Also, some preliminary researches have shown the sperm may damage when passing through the male reproductive tract by decreasing the quality of sperms in terms of DNA integrity (6, 20, 21). We demonstrated that the surgically extracted sperm will do better than ejaculated sperm in ICSI and improve pregnancy outcomes in men with idiopathic cryptozoospermia.