1. Background
There is a new technique to measure total body water (TBW) named bio-electrical impedance analysis (BIA) (1), used in healthy subjects and patients.
The electrical properties of tissue are studied since 1871 (2). These properties are explained with a wide range of frequencies on variety of damaged or undamaged tissue.
BIA principles, which use electrical properties of the body, were developed during the 1970s. This process utilizes the relationship between body water content and its impedance. Recently, to overcome the difference between resistance (R) and body mass, segmental BIA is developed.
2. Introduction
The compositions of body are 60% water, 18% protein, 16% fat, and 6% minerals. Children have more water in their body (70%) compared with adults, while females have less water compared with males (3).
Data show that BIA is a useful technique to evaluate fluid resuscitation in patients with different diseases such as malnutrition, trauma, burns sepsis, and the ones undergoing hemodialysis (4-6). BIA transmits an electric current through the body by using different frequencies and measures resistance, reactance, phase angle, impedance, and capacitance (3, 7). Two or 4 surface electrodes are used to perform a BIA (8, 9).
3. Definitions
The definitions of electrical parameters generated by BIA are as follows:
Capacitance: The storage of an electrical charge by a condenser for a short moment of time. Capacitance is measured in a healthy cell membrane. Electrical capacitance increases or decreases according to the health and number of cells.
Reactance: The opposition of a circuit element to a change in the current or voltage, due to that element’s inductance or capacitance (connected to cell mass).
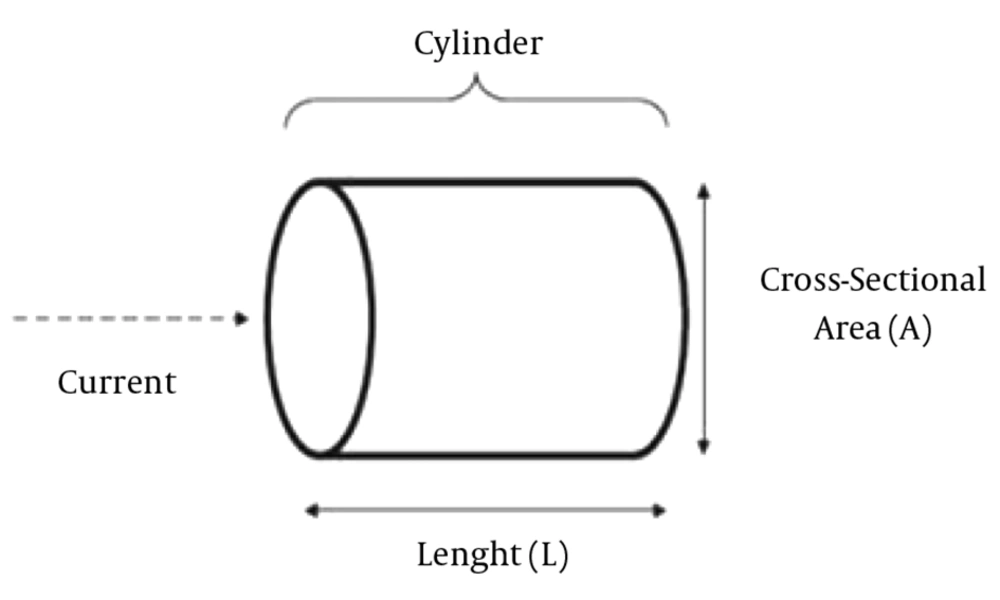
Resistance: The resistance of an electrical conductor is a measure of the difficulty to pass an electric current through that conductor (reversely related to the water content). The resistance of a conductive material is equal to its length (L) divided by its cross section area (A).
Insulator: Tissue that consists of cells that are not conducting electrical signals such as fat cells, where resistance and impedance are high.
Conductor: Tissue that allows electricity to flow easily such as muscles and water, where resistance and impedance are low.
Impedance: Represents the ratio between insulation and conductive tissue.
Frequency: Frequency is the number of repetitions of a complete electric wave form per second (1repetition per second is Hz) (1, 7).
Phase Angle: Phase angle represents the relationship between resistance and reactance.
The human body can be modeled as one central part shown in Figure 1.
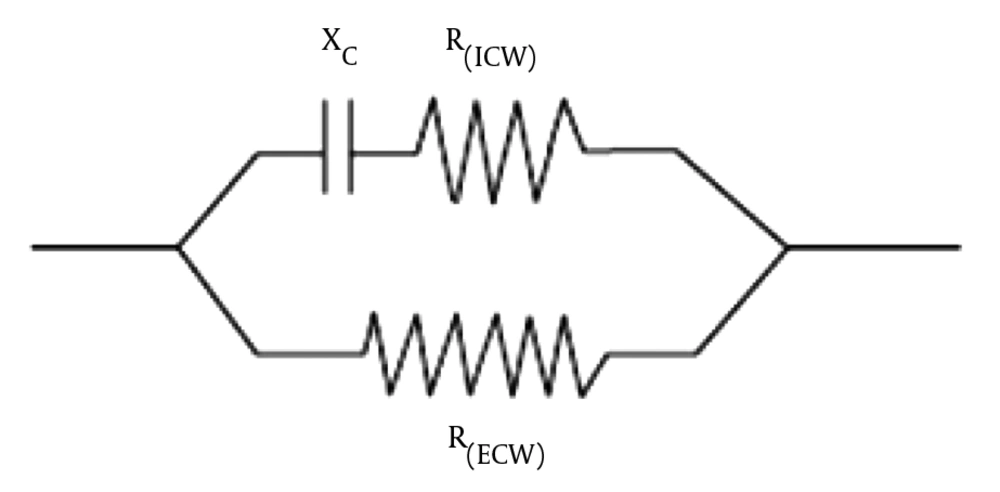
The capacitance (C) rises from cell membrane and resistance (R) comes from intracellular and extracellular fluids. Impedance is a term to explain the combination of capacitance and resistance. Human body consists of several electrical circuits (10), represented by resistors and capacitors connected in series and parallel to explain the actions of tissue. A common circuit which signifies the bioelectrical effect of tissue is resistance of extra cellular water (ECW) in parallel to the capacitance and resistance of intra cellular water (ICW) in series as shown in Figure 2.
Frick’s circuit: Two parallel electrical conductors; R (ECW): H2O-Na; R (ICW): H2)-K; isolated by a cell membrane; tissue acts as a conductor or isolator. The electrical current goes to the line with the lowest resistance (R). The cell membrane of a body contains nonconductive material, 2 lipid layers and conductive protein molecules. Cell membranes work as capacitors (C) when electrical flow is applied.
4. BIA Measurement
Impedance, reactance, resistance and capacitance are different parameters obtained by BIA. These parameters are related to the changes of human body (11).
The BIA measurement is performed by 2 current electrodes that drive electricity through the body and 2 detection electrodes located on hands and feet to detect impedance by changing the frequency of the current through the body (12).
In an insulator tissue, the current could not go through the cell membrane and frequency is zero or low. This causes the current pass through the ECW and resistance of the body becomes zero. In contrast, in a perfect capacitor, very high frequency could go into the cell membrane and the total body resistance would be reflecting the combination of both ECW and ICW. However, the occurrence of multiple dispersions prevents alternating current (AC) currents with zero frequency or very high frequency flowing in the body.
In BIA techniques, resistance, and capacitance are measured by frequency. A current with frequency below 100 Hz measures ECW and does not pass to the cell membrane. Whereas, a current with frequency above 100 Hz measures total body water (TBW) and passes membrane to the cell. Then, ICW is calculated by subtracting ECW from TBW. The principle of BIA measurement is shown in Figure 2. The connection between capacitance and resistance reflect electrical properties of the tissue, which are affected by hydration and nutritional status, and diseases.
It takes an electrical current more time to pass through a cell membrane compared to ECW, this delay time is called phase angle. The greater the proportion of cell membrane, the longer the time delay or phase angle. Also, the higher amount of phase angle means that the proportion of ICW is greater than that of ECW (13).
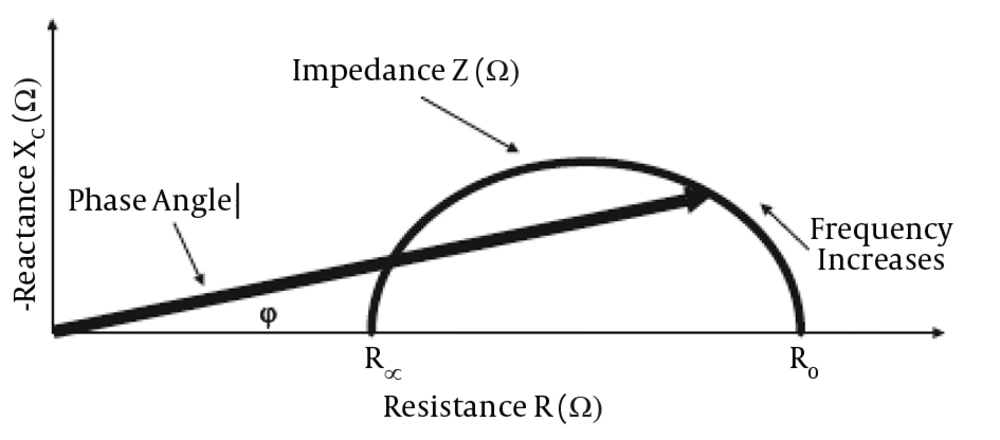
A phase angle of zero degree displays absence of cell membrane and phase angle of 90 degrees displays a capacitive circuit, which indicates membranes.
A high phase angle is observed in healthy individuals, representing a large amount of cell membrane and body cell mass (BCM) with high reactance; whereas a low phase angle is observed in critically ill patients, as presented in Figure 3 (14, 15). By Figure 3, the fluid and nutritional status of patients can be evaluated over time. Some studies showed the relationship among BIA parameters, central venous pressure (cvp), and brain natriuretic peptide (BNP) (16). Raw impedance data can give information about hydration and cell mass integrity (17).
The impedance value, height, weight, gender, and age are 5 essential parameters that are obtained by an accurate BIA measurement. Several methods are developed to measure BIA including: Single frequency BIA, multi-frequency BIA, bioelectrical spectroscopy, segmental BIA, localized BIA, and bioelectrical impedance vector analysis.
Supine position with arms next to the body is the best position to measure BIA. Patients should not be in contact with other persons or objects and electrodes should be correctly placed. Some factors such as infusions of large amounts of normal saline, peripheral edema, skin temperature, and sweating interfere with BIA measurements.
5. BIA Principle
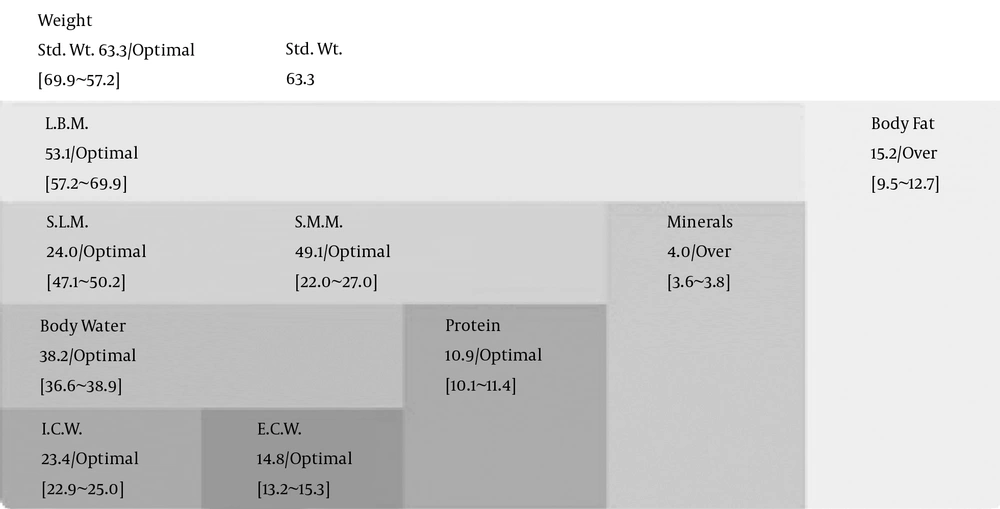
In the best situation, body composition is assumed to be homogenous, but in patients, it is not homogenous. BIA divides composition of the body into 4 compartments of fat, water, mineral, and protein as demonstrated in Figure 4 (13).
ICW: Intracellular water; ECW: Extracellular water, divided into interstitial and intravascular fluid; TBW: Total body water = ICW + ECW; SLM: Soft lean mass = TBW + protein of muscles (skeletal and smooth muscle); SMM: Skeletal muscle mass; LBM: SLM + minerals; BW: Body weight = LBM + body fat; units are expressed in Liters.
6. Validation
BIA is a plain, noninvasive, fast, moveable, and handy method to measure body composition and fluid distribution. However, it is still unclear whether BIA is accurate for patients with critical illnesses (18).
It is necessary to validate the TBW measured by BIA, through other methods such as densitometry. The standard methods to measure body composition and TBW are isotope dilution, dual energy X-ray absorptiometry (DEXA), underwater weighing, portable methods, and air-displacement plethysmography (19, 20).
BIA has a good correlation with DEXA. computed tomography (CT) and magnetic resonance imaging (MRI) can be used to measure abdominal and visceral fat. These techniques require extraordinary services and are not accessible for daily monitoring of body composition.
7. Clinical Applications
To estimate the metabolic status of patients in the intensive care unit (ICU), BIA is a useful technique.
It provides information about changes in body composition and membrane potential at the tissue level by measuring phase angle and fluid imbalance (12).
Also, volume status in patients undergoing hemodialysis, nutritional status, minerals (bone, soft tissue), protein contents of the body, and estimation of glomerular filtration rate (GFR) can be assessed by BIA (21-23).
The clinical usage of BIA could be for patients with burns, cardiovascular diseases, peripheral edema, gastroenteritis, hemodialysis, the continuous veno-venous hemofiltration (CVVH), liver disease, lung disease, acute respiratory distress syndrome (ARDS), malnutrition, bariatric surgery, postoperative fluid status, renal failure, and stroke.
In the early stages of renal failure and in cardio-renal cases, parameters of BIA show lower resistance, abnormal impedance vectors, reduction of phase angle, and higher amount of total body water with a lower body cell mass (24).
The body composition of cases with kidney failure changes and reaches the maximum during the end-stage renal disease. BIA could estimate dry weight and nutritional status of patients undergoing hemodialysis. Also, BIA is helpful to control blood pressure in such patients (25).
In several conditions of critical illness with existence of fluid in their third spaces such as ascites, anasarca, severe peripheral edema, pleural effusion, and massive over hydration, BIA may not provide a good measure of TBW (12). In patients with critical illnesses, the ratio of TBW/FFM is variable and the ratio of body impedance/TBW is often different (3, 12). Therefore, BIA can only be considered as a research tool in patients with critical illnesses.
Oxidative stress and production of reactive oxygen species are responsible for destruction of cell membrane with the consequence of fall in the phase angle (17).
In the normal condition, ratio of ECW/ICW is less than 1. In patients with critical illnesses, this ratio changes as fluid from intravascular migrates to the extra vascular space. Systemic inflammation is responsible for such changes (26).
8. Conclusions
BIA is non-invasive, inexpensive and can be performed at bedside. However, it should be noted that BIA parameters are dependent on the population and the clinical situations may affect this measurement. BIA could assess TBW, ICW, ECW, ECW/ICW ratio, and calculate the volume excess.