1. Background
Amphotericin B (AmB), an amphoteric polyene macrolide, has been considered one of the main options of antifungal therapy for serious and life-threatening mycotic infections for over 50 years despite the introduction of newer antifungal medications such as the azoles (e.g., voriconazole) and echinocandins (e.g., caspofungin), which have better safety profiles (1, 2). This may be due to the fact that azoles and echinocandins are costly, ineffective against certain pathogenic fungi (e.g., Candida krusei and Candida parapsilosis), and not readily available in developing countries such as Iran (3).
Many acute and chronic adverse reactions have been associated with AmB, such as infusion-related reactions (e.g., fever, chills, and hypotension), normocytic normochromic anemia, cardiac toxicity (e.g., ventricular tachycardia and hypertension), hepatic toxicity (e.g., increase in liver enzymes and bilirubin), neurologic toxicity (e.g., confusion, delirium, tremor, and seizure), and nephrotoxicity (4, 5).
Nephrotoxicity is the most clinically significant adverse reaction of AmB (5). Major features of AmB-induced nephrotoxicity include an increased serum creatinine level, a decreased glomerular filtration rate (GFR), urinary potassium wasting, hypokalemia, urinary magnesium wasting, and hypomagnesemia (6). Although it is a common event in clinical settings, different aspects of AmB nephrotoxicity have not been studied well or in detail in our population.
2. Objectives
The purpose of this investigation was to specifically assess the frequency, time onset, and possible associated factors of AmB nephrotoxicity in hospitalized patients in hematology-oncology wards in the southwest of Iran.
3. Patients and Methods
A cross-sectional, observational study was performed over a period of 9 months from August 2015 to April 2016 in 2 hematology-oncology and 1 hematopoietic stem cell transplantation wards of Namazi Hospital, which is affiliated to Shiraz University of Medical Sciences, Shiraz, Iran. The medical ethics committee of the hospital approved the study, and all patients signed and approved a written informed consent form.
The inclusion criteria for recruiting patients were as follows: 1) age 15 years or older; 2) no documented history of acute kidney injury defined by an increase in serum creatinine ≥ 0.3 mg/dL within 48 hours, or an increase in serum creatinine by ≥ 1.5 times the baseline in the previous 7 days, or a urine volume < 0.5 mL/kg/h for 6 hours (7); 3) no documented history of chronic kidney disease (clearance creatinine below 60 mL/min/1.73 m2 calculated by the simplified Modification of Diet in Renal Disease equation); 4) no documented history of peritoneal or hemodialysis for > 3 months (8); 5) no documented history of having received AmB by any administration route in the previous 14 days; and 6) having received any formulation of AmB intravenously for at least 1 week.
The required demographic and clinical data of the patients were recorded. They included age, sex, weight, AmB dose, duration and indication, duration of AmB infusion, type of co-administered medications that may exacerbate or attenuate AmB nephrotoxicity, length of hospital stay, and mortality.
Serum urea and creatinine were monitored daily during the course of the AmB treatment. According to routine ward practice, serum potassium and magnesium were checked daily and once weekly for patients, respectively. Because most cases of AmB nephrotoxicity occur during the first 2 weeks of treatment (9, 10), urine urea, creatinine, sodium, potassium, and magnesium levels were determined at days 0, 3, 5, 7, 10, and 14 of the AmB treatment. Serum as well as urine urea, creatinine, and the aforementioned electrolytes were determined using an Auto-analyzer (Shanghai Xunda Medical Instrument, Shanghai, China). The creatinine levels in the serum and urine samples were established using the modified Jaffe colorimetric reaction.
AmB nephrotoxicity was defined as an estimated ClCr ≥ 50% (calculated by the Cockcroft-Gault formula) or an increase in serum creatinine (double the baseline value) (11). Either the fractional excretion of sodium > 2% or the fractional excretion of urea > 50% (in cases of diuretic co-administration) was considered as acute tubular necrosis (ATN) (12). Serum level potassium and magnesium below 3 mEq/L and 1.2 mEq/L were defined as hypokalemia and hypomagnesemia, respectively (13). A urine potassium-to-creatinine ratio above 13 mEq/g was considered renal potassium wasting (14). The time onset of nephrotoxicity, hypokalemia, and hypomagnesemia after the initiation of AmB was also determined. Daily dose reductions, every other day dosing, discontinuation, or performing dialysis as probable measures of managing AmB nephrotoxicity were recorded.
3.1. Statistical Analysis
Continuous data were expressed as either mean ± standard deviation (SD) or mean ± standard error (SE). Categorical variables were reported as percentages. Probable associations between the categorical variables were evaluated by the chi-squared or Fisher’s exact test. The Fisher’s exact test was applied if more than 25% of the categories had expected frequencies of less than 5. The parametric and non-parametric continuous variables were examined by the independent t- and Mann-Whitney U tests, respectively. Using the “stepwise” method, the logistic regression analysis with the odds ratio (OR) and 95% confidence interval (CI) were employed to determine the associated factors of AmB nephrotoxicity. In the first step, the possible association of each independent variable, namely age, gender, AmB cumulative dose, duration of AmB infusion, baseline GFR value, mean daily oral/intravenous sodium supplementation, and the co-administration of aminoglycosides, calcineurin inhibitors, vancomycin, acyclovir, loop diuretics, corticosteroids, and sodium bicarbonate, with AmB nephrotoxicity (as the dependent variable) was assessed separately by univariate analysis. Those with P values of less than 0.4 were then considered together for the multivariate logistic regression analysis. Statistical significance in all analyses was defined by P values < 0.05, except for the first step of the logistic regression analysis (P values < 0.4). All the above statistical analyses were performed using SPSS version 20.
4. Results
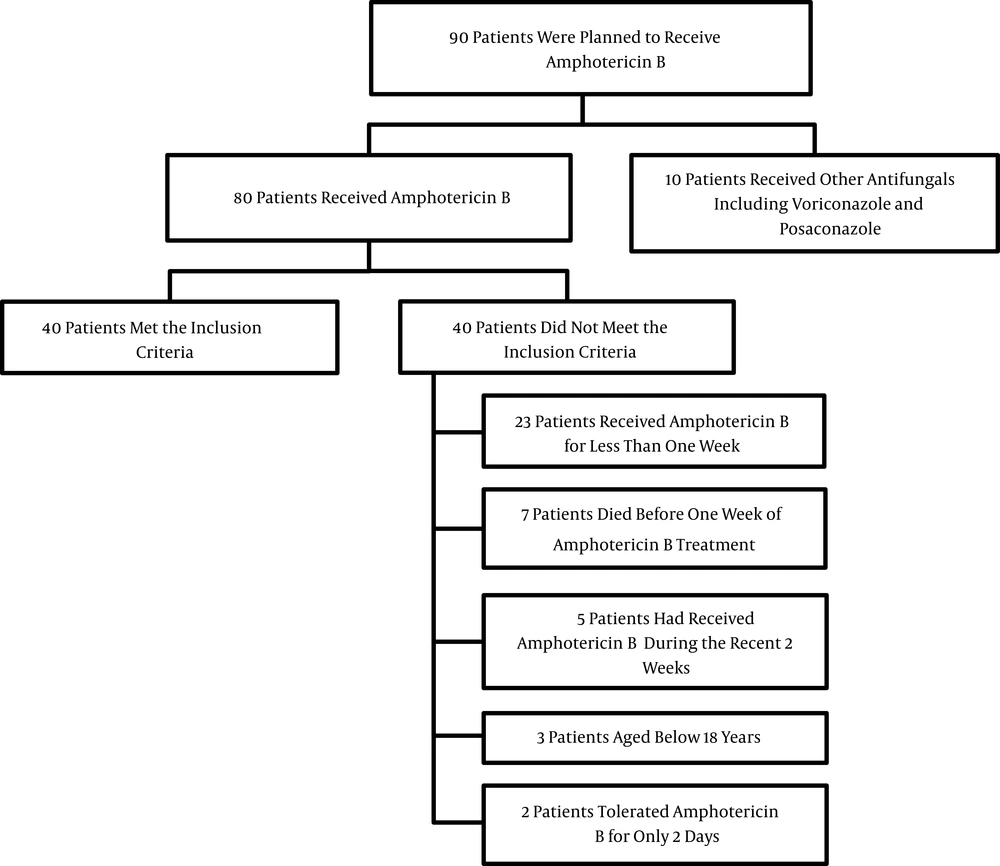
During the 9-month study period, 90 patients who were scheduled to receive AmB were screened. Of these, 40 individuals were eligible for inclusion in the study. A flowchart detailing the reasons patients dropped out of the study is presented in Figure 1.
The mean ± SD age of the study population was 37.95 ± 14.07 years. More than three-fifths (65%) of the cohort were males. The most common admission diagnosis of the patients was acute myeloid leukemia (60%) followed by acute lymphoid leukemia (20%), Hodgkin disease (5%), and aplastic anemia (5%).
Prophylaxis of aspergillosis (52.5%), treatment of aspergillosis (17.5%), and treatment of mucormycosis (12.5%) were the three most frequent indications of AmB administration (Table 1). Twenty-five and 15 patients received liposomal and conventional AmB, respectively. The mean ± SD daily doses of conventional and liposomal AmB were 28.79 ± 10.84 mg and 212.78 ± 99.33 mg, respectively. The mean ± SD cumulative dose of conventional AmB was 223.79 ± 125.53 mg and 2367.15 ± 2137.89 mg for liposomal AmB. All courses of either conventional or liposomal AmB infusions were administered within 2 - 6 hours. The duration of the AmB treatment ranged between 7 and 23 days. Potential nephrotoxic medications, namely acyclovir, corticosteroids, calcineurin inhibitors (cyclosporine), vancomycin, loop diuretics (furosemide), and aminoglycosides (amikacin), were given to 38 (95%), 28 (70%), 21 (52.5%), 19 (47.5%), 5 (12.5%), and 1 (2.5%) patients, respectively. In contrast, spironolactone and sodium bicarbonate as potential nephroprotective agents were administered to 7 (17.5%) and 4 (10%) individuals, respectively. No patient received cisplatin, ifosfamide, cyclophosphamide, dopamine, and mannitol during the study period. The mean ± SD daily oral/intravenous sodium supplementation administered in conjunction with the AmB treatment was 124.75 ± 62.15 mEq.
| Indication | Frequency (%) |
|---|---|
| Prophylaxis of aspergillosis | 21 (52.5) |
| Treatment of aspergillosis | 7 (17.5) |
| Treatment of mucormycosis | 5 (12.5) |
| Treatment of febrile neutropenia | 4 (10) |
| Treatment of visceral leishmaniasis | 2 (5) |
| Treatment of systemic candidiasis | 1 (2.5) |
During the study, 11 (27.5%) and 21 (52.5%) patients developed AmB nephrotoxicity and ATN, respectively. The mean ± SD onset of AmB nephrotoxicity was 6.73 ± 2.36 days. In accordance with the univariate analysis, AmB indication (P = 0.259), vancomycin co-administration (P = 0.388), duration of liposomal AmB infusion (P = 0.141), and amount of oral/intravenous sodium supplementation (P = 0.375) were selected. They were then entered into the multivariate logistic regression model. Based on this model, none of these variables were significantly associated with AmB nephrotoxicity (Table 2).
| Variable | With nephrotoxicity (n = 11) | Without nephrotoxicity (n= 29) | Univariate model | Multivariate model | ||
|---|---|---|---|---|---|---|
| P valuea | OR (95% CI) | P valueb | OR (95% CI) | |||
| Age (years) | 0.87 | 1.004 (0.955 - 1.056) | - | - | ||
| Mean ± SD | 38.54 ± 16.27 | 37.72 ± 13.45 | ||||
| Range | 18 - 62 | 19 - 58 | ||||
| Gender (%) | 0.53 | 0.614 (0.134 - 2.818) | - | - | ||
| Male | 8 (72.73) | 18 (62.07) | ||||
| Female | 3 (27.27) | 11 (37.93) | ||||
| Baseline glomerular filtration rate (ml/min/1.73m2) | 0.51 | 0.519 (0.074 - 3.625) | - | - | ||
| < 90 | 2 (18.18) | 3 (10.34) | ||||
| ≥ 90 | 9 (81.82) | 26 (89.66) | ||||
| Amphotericin B indication (%) | 0.32 | 0.422 (0.076 - 2.341) | 0.259 | 0.182 (0.009 - 3.518) | ||
| Treatment of aspergillosis | 2 (18.18) | 10 (34.48) | ||||
| Others | 9 (81.82) | 19 (65.52) | ||||
| Conventional amphotericin B cumulative dose (mg) | 0.46 | 1.002 (0.996 - 1.009) | - | - | ||
| Mean ± SD | 251.56 ± 171.29 | 212.1 ± 104.24 | ||||
| Range | 140 - 633 | 90 - 500 | ||||
| Liposomal amphotericin B cumulative dose (mg) | 0.38 | 1 (0.999 - 1) | - | - | ||
| Mean ± SD | 2124.96 ± 1789.3 | 2443.63 ± 2275.72 | ||||
| Range | 300 - 4900 | 150 - 6900 | ||||
| Duration of conventional amphotericin B infusion (hr) | 0.88 | 0.907 (0.253 - 3.259) | - | - | ||
| Mean ± SD | 5.75 ± 0.7 | 5.78 ± 0.63 | ||||
| Range | 4 - 6 | 4 - 6 | ||||
| Duration of liposomal amphotericin B infusion (hr) | 0.141 | 0.613 (0.32 - 1.176) | 0.086 | 0.395 (0.137 - 1.14) | ||
| Mean ± SD | 4 ± 1.55 | 5.05 ± 1.433 | ||||
| Range | 3 - 6 | 3 - 6 | ||||
| Co-administration of aminoglycosides (%) | 1 | NAc | - | - | ||
| Yes | 1 (9.09) | 0 (0) | ||||
| No | 10 (90.91) | 29 (100) | ||||
| Co-administration of vancomycin (%) | 0.388 | 1.875 (0.45 - 7.821) | 0.92 | 1.116 (0.13 - 9.574) | ||
| Yes | 4 (36.36) | 15 (51.72) | ||||
| No | 7 (63.64) | 14 (48.28) | ||||
| Co-administration of acyclovir (%) | 0.481 | 2.8 (0.16 - 4.103) | - | - | ||
| Yes | 10 (90.91) | 28 (96.55) | ||||
| No | 1 (9.09) | 1 (3.45) | ||||
| Co-administration of loop diuretics (%) | 0.69 | 1.6 (0.159 - 6.131) | - | - | ||
| Yes | 1 (9.09) | 4 (13.79) | ||||
| No | 10 (90.91) | 25 (86.21) | ||||
| Co-administration of corticosteroids (%) | 0.59 | 1.5 (0.344 - 6.549) | - | - | ||
| Yes | 7 (63.64) | 21 (72.41) | ||||
| No | 4 (36.36) | 8 (27.59) | ||||
| Co-administration of cyclosporine (%) | 0.873 | 0.893 (0.222 - 3.594) | - | - | ||
| Yes | 6 (54.55) | 15 (51.72) | ||||
| No | 5 (45.45) | 14 (48.28) | ||||
| Co-administration of sodium bicarbonate (%) | 0.906 | 1.154 (0.107 - 12.44) | - | - | ||
| Yes | 1 (9.09) | 3 (10.34) | ||||
| No | 10 (90.91) | 26 (89.66) | ||||
| Oral/intravenous sodium supplementation (mEq/day) | 0.375 | 0.99 (0.968 - 1.012) | 0.638 | 0.991 (0.957 - 1.028) | ||
| Mean ± SD | 110.36 ± 38.34 | 130.21 ± 68.88 | ||||
| Range | 51 - 187 | 71.5 - 462 | ||||
aLess than 0.4 was selected for the multivariate regression model.
bLess than 0.05 was considered significant.
cNA, Not available.
AmB nephrotoxicity resolved spontaneously in 5 of the 11 patients who developed the reaction. In contrast, nephrotoxicity in the remaining 6 individuals continued until their deaths. AmB administration was discontinued permanently in 2 individuals who developed nephrotoxicity, and they underwent emergent hemodialysis. There was no statistically significant difference in the duration of hospitalization between the patients with and without AmB nephrotoxicity (27.72 ± 4.81 and 30.33 ± 13.57 days, respectively; P = 0.541). The mortality rate was also comparable between the patients with (54.54%) and without (48.27%) AmB nephrotoxicity (P = 0.723).
Hypokalemia was identified in 18 (45%) of the 40 patients. The frequency of renal potassium wasting during the AmB treatment was 27.5%. The mean ± SD onset of hypokalemia during AmB administration was 5.06 ± 3.35 days. Only one documented episode of hypomagnesemia was detected in the study population during the AmB treatment. Considering the fact that serum magnesium levels were not measured routinely for most of the patients, determining the rate of renal magnesium wasting was not feasible. The co-administration of neither loop (P = 0.642) nor potassium-sparing diuretics (P = 0.211) was significantly associated with hypokalemia. The mean ± SE daily amounts of intravenous potassium and magnesium administered during the course of the AmB treatment were 52.43 ± 12.13 and 3.51 ± 2.04 mEq, respectively.
5. Discussion
The incidence (27.5%) and time onset (5.06 ± 3.35 days) of AmB nephrotoxicity in the present study were within the ranges reported in the literature. Some degree of increase in serum creatinine has been detected within 2 weeks in up to 80% of patients who received AmB (9, 10). In one of the most prominent studies in this regard, a 9-year retrospective analysis demonstrated that 138 (28%) of 494 adult in-patients experienced some type of nephrotoxicity during AmB treatment (10). In a 1-year prospective observational study by Tavakoli-Ardakani et al. (15) in the Hematology-Oncology and Stem Cell Transplantation wards at Taleghani hospital in Tehran, 9 (25.71%) out of 35 patients developed an increase in serum creatinine and BUN during the course of AmB treatment. This rate was reported to be 27.8% at another referral hematology-oncology and stem cell transplantation center in Tehran (16). In the adult infectious diseases ward at Imam Khomeini Hospital in Tehran, Khalili et al. (17) demonstrated that 10 (76.92%) of 13 individuals receiving AmB alone developed acute kidney injury (AKI); however, the incidence of AKI among the patients given AmB along with ceftriaxone and/or vancomycin was 86.68%. The time onset of AmB nephrotoxicity was not generally reported in the aforementioned Iranian studies. The wide variations in the incidence of AmB nephrotoxicity noted above could be due to different study methodologies, clinical settings, relevant risk factors, and definitions. Notably, 62.5% of the individuals in our study were given liposomal AmB, which has a considerably less nephrotoxic formulation. In contrast, only conventional AmB was administered in the other studies. This could partially justify the relatively lower rate of AmB nephrotoxicity in our cohort than in similar studies in Iran.
In the present study, AmB nephrotoxicity in 45.45% of the affected patients resolved spontaneously without any intervention. Only 2 patients in our cohort required emergency hemodialysis due to the severity and persistency of AmB nephrotoxicity. It has been reported that AmB nephrotoxicity is predominantly reversible within a few months after discontinuation of treatment (18); however, about 15% of affected patients may require renal replacement therapy such as dialysis (19). In the Shariati Hematology-Oncology and Stem Cell Transplantation wards, Hayatshahi et al. (16) showed that AmB nephrotoxicity led to a dose reduction of this agent in 3.7% of cases. In terms of clinical outcomes, mortality and duration of hospitalization were comparable between patients with and without AmB nephrotoxicity in the present population. In contrast, Bates et al.’s (20) study of a large population (707 adults) in the United States reported that acute renal failure due to AmB increased the mean length of hospital stay by 8.2 days (P < 0.0001) and increased the total cost of treatment by $29,823. The mortality rate was also much higher (54% vs. 16%; P = 0.001). Differences in the confounding factors (e.g., the severity of the underlying disease), the type of AmB formulation used (conventional vs. lipid-based), and sample size could partially account for these disparities.
We considered P values greater than 0.05 (0.4) in the univariate analysis to select all possible variables linked to the dependent variable. Among the different demographic, clinical, and paraclinical features, AmB indication, vancomycin co-administration, duration of liposomal AmB infusion, and amount of oral/intravenous sodium supplementation were identified as factors associated with AmB nephrotoxicity. Nevertheless, none of these variables were significantly associated with AmB nephrotoxicity in the multivariate logistic regression model. Several large-scale retrospective and prospective studies have identified males, an average daily dose of AmB above 35 mg, a cumulative dose of AmB greater than 2 - 5 g, dehydration, co-administered diuretics and nephrotoxic agents (e.g., aminoglycosides, cyclosporine, foscarnet, cisplatin, and ifosfamide) or corticosteroids, and baseline renal dysfunction as potential risk factors for AmB nephrotoxicity (21-23). Inadequate statistical power resulting from the relatively small sample size and the relatively low incidence of AmB nephrotoxicity due to the administration of liposomal AmB appear to be the main reasons for our findings in this regard. It has been suggested that conventional AmB should preferably not be administered to patients with two or more of the aforementioned risk factors of AmB nephrotoxicity (10, 24). Notwithstanding, with the introduction of considerably less nephrotoxic formulations of AmB into the market, this suggestion does not seem to make sense in clinical practice.
During the course of the AmB treatment in the current study, hypokalemia and renal potassium wasting developed in 45% and 27.5% of the patients, respectively. In contrast, hypomagnesemia was detected in only 1 patient (2.5%). The incidence of AmB-induced hypokalemia in our cohort was lower than that reported in the literature (75% - 90%) (25). This may be due to the fact that 62.5% of the patients in the present study received liposomal AmB. Several publications have estimated the frequency of AmB-induced hypomagnesemia to be between 15% and 100% depending on the dose and formulation of AmB (26). Apart from the type of AmB formulation and the presence of relevant risk factors, such as the co-administration of loop and potassium-sparing diuretics, the low rate of hypomagnesemia in our population could have been because the patients’ serum magnesium levels were measured only once a week during the AmB treatment in the studied wards. Accordingly, calculating renal magnesium wasting was not feasible. Severe hypokalemia and hypomagnesemia due to AmB can cause metabolic complications, rhabdomyolysis, and life-threatening arrhythmias (25); however, these adverse events were not observed in our cohort at least during the course of the AmB treatment.
In conclusion, nearly one-third (27.5%) of our cohort developed nephrotoxicity within the first week of AmB treatment. AmB nephrotoxicity resolved spontaneously in about half (45.45%) of the affected patients without any intervention. Mortality and the duration of hospitalization were comparable between patients with and without AmB nephrotoxicity. No studied demographic, clinical, and paraclinical features of the study population were significantly associated with AmB nephrotoxicity. Among the studied electrolyte abnormalities, hypokalemia and renal potassium wasting were the most notable, affecting about one-half and one-third of AmB recipients, respectively. The co-administration of either loop or potassium-sparing diuretics did not significantly affect electrolyte abnormalities during the course of the AmB treatment. Close monitoring of renal function indexes, including serum creatinine, BUN, serum potassium, and magnesium, during AmB treatment is highly recommended. Additionally, implementing approved prophylactic measures, such as saline loading (150 mEq/day) before and/or during AmB infusion, and exploiting lipid-based formulations of AmB (especially liposomal), if available and affordable, should be considered to minimize the possibility of AmB nephrotoxicity.