1. Background
Prostate cancer is the most commonly diagnosed cancer after skin cancer; it is the sixth most common cause of cancer death throughout the world and second in the United States (1, 2). According to recent studies, prostate cancer is the second most common cancer among Iranian men (3).
Prostate cancer has various risk factors, including old age, family history, and black ethnicity (4). This cancer progresses from prostatic intraepithelial neoplasia (PIN) to localized, locally advanced, and finally metastatic cancer (5). Radical prostatectomy has been considered the gold standard treatment for patients with clinically localized prostate cancer (6).
Some patients with prostate cancer show a higher clinical stage after radical prostatectomy (7), including lymph node invasion (LNI), seminal vesicle invasion (SVI), and extraprostatic extension (EPE), which may affect cancer prognosis, recurrence rate, and survival. Patients with adverse pathologic features after radical prostatectomy need adjuvant therapy, such as radiotherapy or hormone therapy (7). Gleason score, PSA density, and the PSA velocity are some of the parameters used to predict adverse pathologic features (8).
The aim of the present study was to evaluate the prognostic value of PSA density and Gleason score in predicting up-staging after radical prostatectomy in patients with clinically localized prostate cancer.
2. Objectives
The aim of this study was to evaluate the prognostic value of PSA density and Gleason score in predicting adverse pathologic features in patients with localized prostate cancer who undergo radical prostatectomy.
3. Methods
We conducted a cross-sectional study of 105 patients with localized prostate cancer who underwent radical prostatectomy between 2006 and 2013. The exclusion criteria were neoadjuvant therapies, such as radiotherapy, chemotherapy, or hormone therapy. From the patients’ medical files, we collected Gleason scores and PSA levels, in addition to the results of pathological evaluations after radical prostatectomy, including prostate dimension, stage, LNI, SVI, and EPE. PSA density was calculated by dividing the serum PSA level by the prostate volume (maximum longitudinal diameter × maximum transverse diameter × maximum AP diameter × π/6).
The data were analyzed using SPSS version 21.0. We used the t-test to compare quantitative variables. When the data were not normally distributed, we used the Mann-Whitney U-test. ROC curve analysis was also performed to compare the discrimination power of various parameters in determining high-risk disease.
4. Results
The mean PSA density was 0.27 (0.17 SD). The frequencies of EPE, SVI, and LNI were 21.9, 16.2, and 2.9, respectively (Table 1). The Mann-Whitney U-test demonstrated a significant correlation between PSA density and adverse pathologic features (EPE, SVI, and LNI); however, we did not find any association between Gleason score and LNI (Table 2).
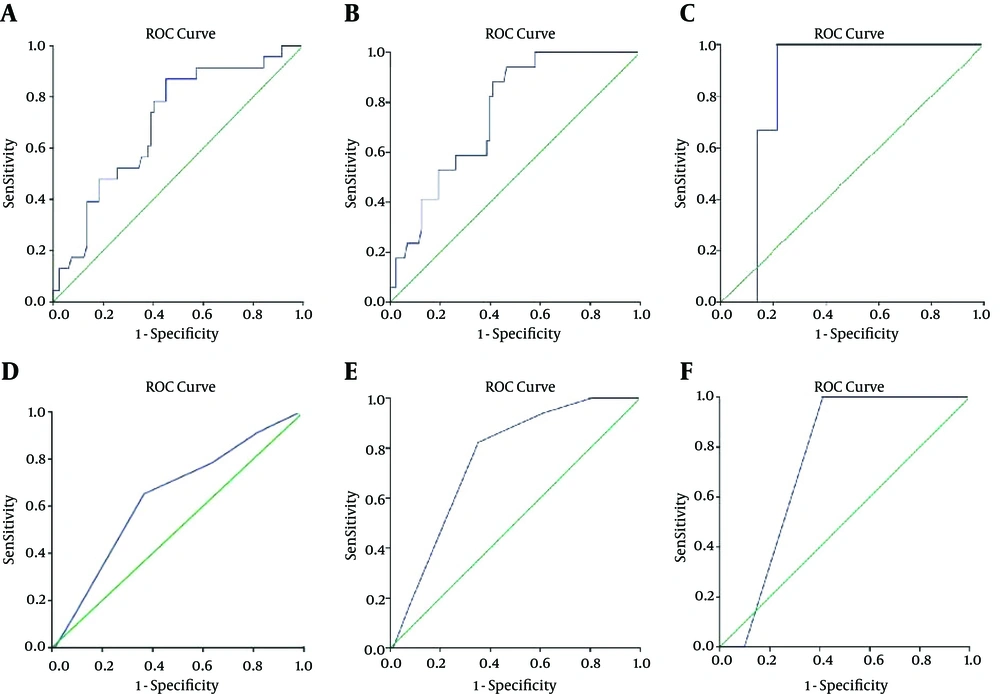
The area under the curve (AUC) in the ROC analysis, showing the relationship between PSA density/Gleason score and adverse pathologic features, is shown in Figure 1A-F. The sensitivity and specificity of PSA density and Gleason score in predicting adverse pathologic features are shown in Table 3.
| Mean | SD | Median | |
|---|---|---|---|
| PSA Density | 0.27 | 0.17 | 0.2 |
| Gleason Score | 6.03 | 1.45 | 6 |
| EPE, No. (%) | 23 (21.9) | ||
| SVI, No. (%) | 17 (16.2) | ||
| LNI, No. (%) | 3 (2.9) |
| PSA Density | Gleason Score | |
|---|---|---|
| EPE, No. (%) | 0.004 | 0.038 |
| SVI, No. (%) | 0.001 | 0.001 |
| LNI, No. (%) | 0.045 | 0.160 |
| PSA density | Gleason score | |
|---|---|---|
| EPE | 86 - 55 | 78 - 37 |
| SVI | 88 - 60 | 94 - 39 |
| LNI | 100 - 79 | NS |
5. Discussion
Various preoperative parameters, such as PSA, PSA-related parameters, and prostate biopsy-based Gleason scores, may be predictive of tumor stage in prostate cancer. Since the incidence of this disease has increased as a consequence of widespread PSA screening, more patients are undergoing radical prostatectomy. However, some patients might have locally advanced or even systemic disease, including EPE, SVI, or LNI. All of these are poor prognostic factors and are predictive of higher rates of biochemical failure, recurrence, and diminished survival. Most patients who meet these conditions may benefit from a neoadjuvant or adjuvant treatment protocol. Therefore, it is of value to improve the accuracy of staging prior to the performance of radical prostatectomy. This is also useful for surgical planning and for deciding whether to proceed with sparing the neurovascular bundle, which carries the intrinsic dangers of positive surgical margins or incomplete tumor removal in patients with locally advanced or high-risk disease. Therefore, different preoperative factors, both clinical and pathological, are of utmost importance for predicting advanced prostate cancer and providing benefits for patients.
The most frequently applied nomograms for predicting pathological stage are the Partin tables, which estimate the probability of EPE, SVI, and LNI. These nomograms use clinical stage, PSA, and biopsy Gleason scores as preoperative factors to predict adverse pathology. These tables are of value in determining treatment protocols. Few studies have focused on the correlation of PSA density and EPE, SVI, and LNI, and the results are controversial. In the present study, we showed that PSA density has the potential to accurately predict up-staging in patients with clinically localized prostate cancer. Kundu et al. (9), in order to survey the association between PSA density and the invasion potential of prostate cancer, studied 1,662 patients, classifying them into four groups based on PSA density (< 0.1, 0.1 - 0.14, 0.15 - 0.19, and > 0.20). They reached clear surgical margins in 82%, 75%, 75%, and 55% of these patients, respectively (P < 0.001). They similarly concluded that PSA density is strongly correlated with adverse pathologic features. Another study performed by Freedland et al. (10) on 325 patients with prostate cancer identified preoperational parameters that are predictive of recurrence after radical prostatectomy. They determined that PSA density is a strong predictor of EPE, positive surgical margins, and SVI, and that it is more accurate compared to PSA. We noted a similarly high accuracy of PSA density as a predictor.
Radwan et al. (11), in a similar study, calculated prostate volume by two methods (transrectal ultrasonography and postsurgical direct measurement). The overall results were similar to ours and showed that PSA density may accurately predict adverse pathologic features after radical prostatectomy.
Brassell et al. (12) compared PSA with PSA density in order to predict tumor mass volume, margin, stage, and recurrence. Ou et al. (13) conducted a similar study and included patients with T1c prostate cancer who had undergone radical prostatectomy. Both of these studies showed that PSA density is superior to PSA for predicting EPE.
Horninger et al. (14) indicated that PSA, as a predictor of EPE among patients with prostate cancer, is stronger than PSA density if the Gleason score is ≥7. Sfoungaristos et al. (15) believed that the Gleason score had no significant correlation with LNI. Our study indicated similar results.
In conclusion, attention should be paid to PSA, PSA density, and Gleason score alongside each other in order to more accurately predict the adverse pathologic features of prostate cancer.