1. Background
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic nephropathy characterized by the presence of fluid-filled cysts primarily in the kidneys. This disease is one of the most common hereditary disorders in humans. The incidence of ADPKD is about 1 in 500 to 1 in 1000 in western countries, however, there is no updated and comprehensive data regarding the polymorphic linkage markers pattern among the Iranian population. Up to 50% of patients with ADPKD require renal replacement therapy by 60 years (1). The frequency of ADPKD is high as compared to other prominent genetic disorders, approximately 10, 15, and 20 times higher than sickle cell anemia, cystic fibrosis, and Huntington’s disease, respectively. Another form of PKD is autosomal recessive and rare with an incidence of 1 in 20,000 (2).
ADPKD is genetically heterozygous and the mutations occur in the PKD1 gene located on chromosome 16p13.3 in 85% of the patients (3) and in PKD2 gene on chromosome 4q21-23 in 15% of the patients (4). The presence of the third gene related to ADPKD in some patients is unclear and does not show linkage to either the PKD1 or PKD2 genes (3, 4). The mutations of PKD1 and PKD2 genes can produce identical renal and extrarenal manifestations. PKD2 patients develop the above symptoms at a later age with less intensity as compared to PKD1 patients (1).
The PKD1 is a long gene with 46 exons (750 Kbps length), 970 known pathogenic mutations, and there are many homology regions with another part of the genome that make it a hard case for mutation detection. However, PKD2 has a shorter size, which has fewer problems for direct screening for mutation in compare to PKD1. Beyond this, the recently wide progress in sequencing methods has eliminated this problem, however, using a former method like polymorphic microsatellite linkage analysis is acceptable and cheap. The ADPKD screening and predict pre-symptomatic prenatal ADPKD by use of microsatellite markers closely linked to these loci is commonly used in clinical genetics (5-7).
In this regard, we performed this analysis to assess the genetic linkage of 4 microsatellite markers of polycystic kidney disease genes (PKD1 & PKD2) in ADPKD among 18 Iranian affected families living in Yazd.
2. Methods
2.1. Cases and Controls
The samples were collected from 94 individuals of 18 unrelated ADPKD affected Iranian families (from Yazd) in 2016. This familial case-control study had at least 2 affected and 2 - 3 unaffected members from every family, although most of the families were larger. A total of 47 of the introduced patients by Yazd renal diseases charity foundation, who had a confirmed diagnosis considering their special conditions such as age of the patients (29.04 ± 23.76 years), family history, clinical symptoms, related therapies, and number of cysts in the kidney, were entered into our study (Table 1). The genetic pedigrees were drawn based on a completed questionnaire of each patient. The Ethics Committee of Yazd Reproductive Science Center approved the study protocol and the signed consent after ensure understanding were obtained from all participants (ethic code: IR.SSU.RSI.REC.1395.34).
| Characteristics | No. (%) |
|---|---|
| Gender | |
| Women | 19 (40.5) |
| Men | 28 (59.5) |
| Family History | |
| Positive | 45 (95.8) |
| Negative | 2 (4.2) |
| Death | |
| Positive | 39 (83) |
| Negative | 8 (17.1) |
| Clinical symptom | |
| Cyst in both kidneys | 43 (91.5) |
| Kidney stone | 18 (38.3) |
| Blood pressure | 26 (55.3) |
| Renal pain | 29 (61.7) |
| Blood in the urine | 14 (29.8) |
| Treatment | |
| Drug treatment | 20 (42.5) |
| Dialysis | 10 (21.3) |
Demographic and Clinical Data
2.2. Microsatellites Genotyping
Genomic DNA was extracted from peripheral blood leukocytes using the phenol-chloroform method. The quality and quantity of the extracted DNA were checked by NanoDrop spectrophotometer (ND-1000) and gel electrophoresis.
Four microsatellite markers on NCBI map viewer and uniSTS data on chromosome 4q and 16p were selected and separated from each other by ∼10 cM (KG8, 16AC2.5, D4S423 and D4S231) that the type of these markers is (AC)n repeated (5-8). Using the Ensemble genome database, we checked the correct sequence and distance of the STR marker and disease loci. Two microsatellite markers (16AC2.5 and KG8) are localized within 350kb of the PKD1 gene (5, 9-11). D4S423 and D4S231 (specific markers) were used to assess linkage to PKD2 (5, 12, 13).
The primers used for PCR were: KG8 (IF: CTCCCAGGGTGGAGGAAGGTG, IR: GCAG GCACAGCCAGCTCCGAG) (109 - 126 N), 16AC2.5 (IF: AAGGCTGGCAGAGGAG GTG, IR: CAGTTGTGTTTCCTAATCGGCG) (109 - 142 N), D4S423 (IF: TTGAGTAGTTCC TGAAGC AGC, IR: CAAAGTCCTCCATCTTGAGTG (103 - 121 N), D4S231 (IF: ACTATTCAGT GCTAGGAGTTCCC, and IR: GCATCAACTTGGGGAGATCC) (144 - 157 N). Briefly, the target sequence was amplified in 20 μL reaction cocktail containing Mix Red-Mgcl2 (1.5 mM, Amplicon), an equal amount of forward and reverse primer (0. 5 pmol/μL) and genomic DNA (25 ng). Cycle amplification was performed in the Master Cycler (Eppendorf 5331, Germany), (5 minutes at 95°C as an initial denaturation, 35 cycles of 30 seconds at 95°C, 1 minute at an annealing temperature (64°C for KG8, 67°C for D16S291 and D4S423, 58 °C for D4S231), 1 minute at 72°C and 10 minutes at 72°C for final extension). 5 mL of mixture was loaded on a 12% polyacrylamide gel containing 0.5 × TBE buffer for resolving the markers. The gel was run for 5 hours with 100 voltage; after electrophoresis and colored by silver nitrate, the gel was scanned and the allele patterns were analyzed manually. The size of alleles and the informative marker are different in different populations and the sample size and number of evaluated markers are effective in the detection and selection of the informative marker.
2.3. Linkage Analysis and Haplotype Construction
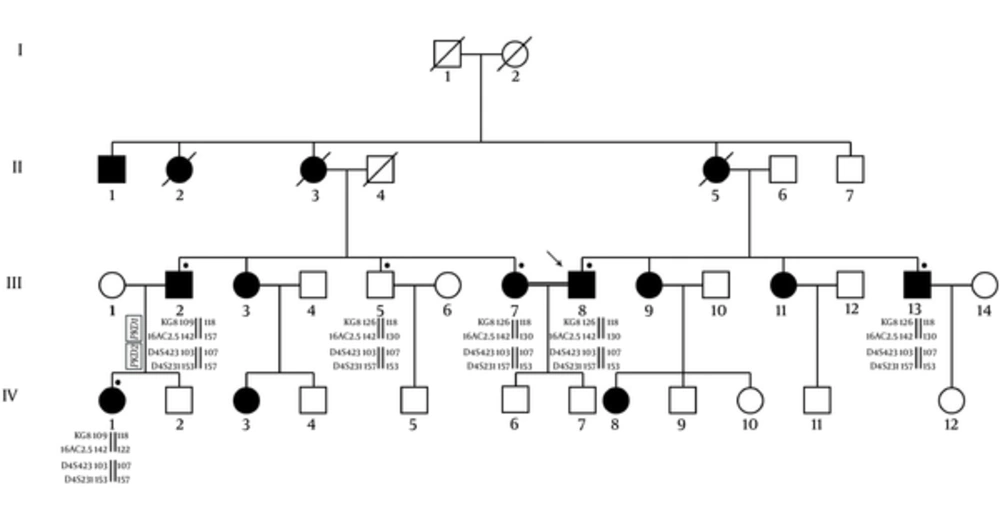
Due to more suitability for a genetic linkage study, the large families were investigated and the evidence of the linkage to PKD1 and PKD2 loci was obtained by using of flanking microsatellite polymorphisms. The calculation of LOD scores for this two-point linkage analysis was done via the FASTLINK software. The resultant data in each family were integrated by means of Bayesian weighting formula for the likelihood estimation of a family to one or other locus (14). The genotyping of individuals includes descendent and married-in patients, where extracted and the founders of genotypes were reconstructed. The relative location of PKD1 and PKD2 satellite markers are illustrated in Figure 1. The haplotypes of them in members of ADPKD families were also shown in Figure 2.
3. Results
D4S423, D4S231, and 16AC2.5 show significant linkage with PKD2 and PKD1, respectively.
The heterozygosity of 18 unrelated families was analyzed. Sixteen different sizes of alleles were seen in 4 markers as presented in Tables 2 and 3. According to the findings 16AC2.5, D4S423, and D4S231 markers are suitable for linkage analysis. There were 5 alleles found for 16AC2.5 marker in our understudied population. The HET and PIC values for these markers were also determined 0.782 and 0.798, respectively. Our finding shows D4S423 and D4S231 had both, 4 alleles. Their HET values were 0.840 and 0.775 and their PIC values were 0.807 and 0.741, respectively. Based on our findings, the evaluated microsatellite markers had the acceptable range for HET and PIC values in our linkage analysis on PKD1 and PKD2 among the ADPKD patients (Table 2). The LOD scores of our linked microsatellite markers to PKD1 and PKD2 were determined. This two-point linkage analysis showed these markers were informative and located at the most flanking locus in each family. The θ values of the maximum LOD scores are also presented in Table 4.
Number and Size of Alleles, heterozygosity of Polymorphic Markers Linked to PKD1 and PKD2 in the Iranian Population
| Genes and Alleles | No. (%) |
|---|---|
| PKD1 | |
| KG8 | |
| 109 | 27 (57.5) |
| 118 | 16 (34.1) |
| 126 | 4 (8.4) |
| 16AC2.5 | |
| 109 | 2 (4.2) |
| 113 | 3 (6.4) |
| 122 | 3 (6.4) |
| 130 | 15 (31.9) |
| 142 | 24 (51.1) |
| PKD2 | |
| D4S231 | |
| 103 | 2 (4.2) |
| 107 | 2 (4.2) |
| 117 | 14 (29.8) |
| 121 | 29 (61.8) |
| D4S423 | |
| 144 | 23 (48.9) |
| 148 | 11 (23.4) |
| 153 | 7 (14.9) |
| 157 | 6 (12.8) |
The Frequency of the Marker Alleles Linked to PKD1 and PKD2 in the Iranian Population
| LOD Scores | ||||||||
|---|---|---|---|---|---|---|---|---|
| Markers | θ = 0.0 | θ = 0.01 | θ = 0.03 | θ = 0.05 | θ = 0.07 | θ = 0.1 | θ = 0.15 | |
| PKD1 | KG8 | 0.28 | 0.28 | 0.27 | 0.26 | 0.25 | 0.23 | 0.20 |
| 16AC2.5 | 2.65 | 2.19 | 1.51 | 1.14 | 0.90 | 0.76 | 0.65 | |
| PKD2 | D4S423 | 4.25 | 4.17 | 3.80 | 3.52 | 3.22 | 2.48 | 0.98 |
| D4S231 | 3.85 | 3.25 | 2.90 | 2.45 | 2.20 | 1.35 | 0.67 | |
Pairwise Z Values for Linkage Between ADPKD and Chromosome 4 Markers
3.1. KG8 Shows Insignificant Linkage with PKD1
The alleles of KG8 marker were 3 forms that the HET and PIC values for this marker were 0.340 and 0.329, respectively. These qualities for HET and PIC were in an acceptable range, however, the calculated LOD and θ values of the maximum LOD scores were not signified regarding KG8 linkage to PKD1 (Table 4).
3.2. The Linkage Analysis and Genotype of the ADPKD Families
The LOD scores and allele segregation analysis showed the linkage to PKD1 was possible approximately 77.8% in 10 families (pedigrees K1-K4, K6, K7, K10-K13, K16, and K17) and this possibility for PKD2 was 16.7% in 7 families (pedigrees K3, K8, K9, K14, K15 and K18).
LOD scores K5 family were mostly negative and linkage to neither of the 2 PKD loci could be assumed. All families with linkage to PKD1 and PKD2 or to either PKD1 or PKD2 were selected for screening the further mutation (Table 5).
| Families | PKD1 | PKD2 | |||
|---|---|---|---|---|---|
| Zmax | θmax | Zmax | θmax | Genotypes | |
| K1 | 0.402 | 0.00 | -1.21 | 0.099 | PKD1 |
| K2 | 0.450 | 0.00 | -4.00 | 0.005 | PKD1 |
| K3 | -0.942 | 0.00 | 1.42 | 0.005 | PKD2 |
| K4 | 0.308 | 0.00 | -1.48 | 0.019 | PKD1 |
| K5 | ∞- | 0.00 | -1.45 | 0.015 | - |
| K6 | 0.720 | 0.014 | -2.45 | 0.013 | PKD1 |
| K7 | -0.608 | 0.00 | -1.43 | 0.015 | PKD1 |
| K8 | -0.905 | 0.00 | 0.112 | 0.125 | PKD2 |
| K9 | -0.306 | 0.00 | 3.89 | 0.006 | PKD2 |
| K10 | 0.698 | 0.00 | -3.00 | 0.113 | PKD1 |
| K11 | 0.567 | 0.014 | -2.05 | 0.007 | PKD1 |
| K12 | 1.20 | 0.00 | -5.80 | 0.004 | PKD1 |
| K13 | 0.725 | 0.00 | -2.60 | 0.015 | PKD1 |
| K14 | -0.603 | 0.00 | 0.115 | 0.013 | PKD2 |
| K15 | -0.305 | 0.00 | 3.20 | 0.019 | PKD2 |
| K16 | 0.502 | 0.014 | -3.00 | 0.006 | PKD1 |
| K17 | -0.935 | 0.00 | -3.15 | 0.014 | PKD1 |
| K18 | -1.30 | 0.00 | 4.00 | 0.088 | PKD2 |
Linkage Analysis and Genotype in 18 Iranian ADPKD Families
4. Discussion
The main part of PKD patients has composed of mutation carriers of PKD1 and PKD2 genes that these genes are responsible for ADPKD in approximately 85% and 15% of cases, respectively. The percentage of non-autosomal dominant PKD patients is also less than 10% among different populations (3, 4). Several studies from around the world report similar results as mentioned above; for example Mizoguchi et al. in 21 Japanese ADPKD families, including 96 individuals and 57 affected members, reported that 17 families (81%) had linkage to PKD1, 2 families (10%); PKD2 and 2 families did not have linkage to either PKD1 or PKD2 (14). Another study was performed by colleagues on 48 Korean families that the results were composed of PKD1 (79%) and PKD2 (21%) (15). Moreover, the similar rate of the genetic heterogeneity has been shown in other populations, such as Argentinians (91%) (16), Bulgarians (73%) (17), and Caucasians (81%) (18). Radpour et al. study, the closest one to our investigation, evaluated 15 Iranian families and reported that the proportion of families linked to PKD1, PKD2, or to other genes was 73%, 13%, and 13%, respectively (5).
Our allele frequencies of PKD1 and PKD2 markers (16AC2.5, KG8, D4S423 and D4S231) were not similar to earlier reports in Caucasian ethnics (8, 10, 19-21), however, our results were compliance with Radpour et. al in an Iranian population (5). For instance, among Spanish ADPKD families (48 ADPKD-affected families), it was reported that 7 alleles for D4S231 (HET: 0.71, Zmax: 4.28) as well as 9 for D4S423 (HET: 0.83, Zmax: 9.03) markers for linkage to PKD2 (8) and 8 different alleles for the KG8 marker and 10 alleles for 16AC2.5 for linkage to PKD1 (19). There is also another study on 30 Hungarian ADPKD-affected families that reported 12 alleles for D16S663 marker, while 16AC2.5, KG8, D4S1563, and D4S2462 had 10, 8, 12, and 11 alleles, respectively (21).
In summary, according to the results, D4S423 (HET: 0.84, PIC: 0.80), D4S231 (HET: 0.77, PIC: 0.74) and 16AC2.5 (HET: 0.78, PIC: 0.79) had the highest heterozygosity rates as well as PIC values and were the most informative markers for PKD1 and PKD2 loci to diagnose ADPKD while the less informative marker was KG8 (HET: 0.34, PIC: 0.32) for PKD1 locus in the population. Therefore, 1 marker linked to the PKD1 gene (16AC2.5) and the 2 markers linked to PKD2 genes (D4S423 and D4S231) were informative for screening of ADPKD patients in our population.