1. Background
Proliferative extra-capillary glomerulonephritis (EGN) or crescentic glomerulonephritis (CGN) is not a specific disease, yet a histological manifestation of severe glomerular damage. The term “extra-capillary proliferation” is used to designate the cellular and/or fibrous proliferation that occupies the Bowman’s space, arising from its capsule. “Extra-capillary” indicates that proliferation occurs outside of the capillary tuft. There is no universal agreement on the percentage of involved glomeruli to diagnose crescentic GN, yet the most commonly used percentage is ≥50%. The definition of a crescent is the presence of at least 2 layers of cells totally (circumferential) or partially (circumscribed) filling the Bowman’s space (1). The clinicobiological presentation is rapidly progressing glomerulonephritis (RPGN) characterized by glomerular syndrome with a loss of kidney function over a period of weeks to months (1, 2). Crescentic glomerulonephritis is a diagnostic and therapeutic emergency. The prevalence of CGN consists between 2% and 10% of all nephrological disorders (3). In sub-Saharan Africa, a few studies have been done on CGN. The aim of this study was to evaluate the clinical/biological, therapeutic, and evolutionary patterns of extra-capillary glomerulonephritis and to identify factors responsible for a poor prognosis.
2. Methods
This was a retrospective descriptive and analytical study based on a renal biopsy registry and the medical records from the nephrology department of Aristide Le Dantec university hospital in Dakar. The study was conducted over a period of five years, from January 1st, 2010 to December 30th, 2015. All reports on kidney biopsies concluding on extra-capillary glomerulonephritis (ECGN) were included. Clinical/biological, etiological, and therapeutic characteristics were identified.
The evolutionary features were collected and analyzed in order to establish factors responsible for a poor renal prognosis. After comparing the clinical, biological, histological, and etiological data, patients were divided to 2 groups (A and B) to assess factors responsible for a poor renal prognosis: group A with GFR ≥ 60 mL/minute after 3 months of treatment and group B with persistent kidney failure (CKD). The researchers divided these patients to 2 subgroups:
- Subgroup B1: those who evolved towards End-Stage Renal Disease (ESRD)
- Subgroup B2: those with stage 3 and 4 CKD
The data were analyzed using statistical package for social sciences (SPSS) software version 18. The averages and percentages were compared using Student’s t test, χ2 test, and Fischer’s exact test, according to their conditions of applicability. Any difference of less than 0.05 was deemed statistically significant.
3. Results
During the study period, 750 biopsies were performed. Forty cases of crescentic glomerulonephritis were found with a prevalence of 5.33%. The mean age was 33.9 ± 16.65 years with a female predominance (26 females/14 males), i.e. a male: female gender ratio of 0.53. The mean time from first symptoms to admission in care unit was 40 ± 29.66 days. Upon admission, 26 (65%) patients presented high blood pressure and 85% had renal insufficiency. Oligoanuria was present in 25 (62.5%) patients. The mean proteinuria was 3.22 ± 2.78 g/24 hour. This was associated with microscopic hematuria in 80% of cases. Thus, the most commonly encountered clinical/biological presentation was rapidly progressive glomerulonephritis (Table 1).
| Parameters | Total Patients (n = 40) |
|---|---|
| Mean age | 33.9 ± ans |
| Male | 14 |
| Female | 26 |
| Mean time from first symptoms to admission, jours | 40 ± 29.66 |
| Clinical symptoms | |
| Hypertension | 26 (65) |
| Oligoanuria | 25 (62.5) |
| Macroscopic hematuria | 4 (10) |
| Cardiac manifestation | 34 (85) |
| Pulmonary manifestation | 25 (62.5) |
| Otorhinolaryngological manifestation | 4 (10) |
| Articular manifestation | 12 (30) |
| Neurological manifestation | 18 (45) |
| Laboratory tests | |
| Mean proteinuria, g/24h | 3.22 ± 2.78 |
| Microscopic hematuria | 32 (80) |
| renal insufficiency at admission | 30 (75) |
| Mean blood creatinine, mg | 61.45 ± 50.87 |
| Cellular crescents | 24 (60.5) |
| Histological lesion | |
| Fibrous crescents | 13 (32.5) |
| Tubulars lesions | 40 (100) |
| Tubular necrosis | 20 (50) |
| Tubular atrophy | 20 (50) |
| Interstitials lesions | 33 (82.5) |
| Interstitial infiltration | 25 (62.5) |
| Fibrosis | 8 (20) |
| Vascular lesions | 22 (55) |
Histologically, the mean duration from admission to renal biopsy was 4.61 ± 4.66 days. By light microscopy, crescents were cellular in 60.5% of cases, fibro-cellular in 57.5%, and fibrous in 32.5% of cases. Completely sclerotic glomeruli were found in 5.15% of cases. Immunofluorescence was performed in 11 patients; 3 showed linear deposits, 4 had granular deposits and 4 had no deposits. Concerning the associated lesions, tubular involvement was present in all patients. Tubular necrosis was seen in 20 (50%) patients and tubular atrophy was observed in 20 (50%) patients. Interstitial involvement was present in 33 (82.5%) patients, with interstitial infiltration in 25 (62.5%) patients and fibrosis in 8 (20%) patients. Vascular involvement was found in 55% of patients (Table 1).
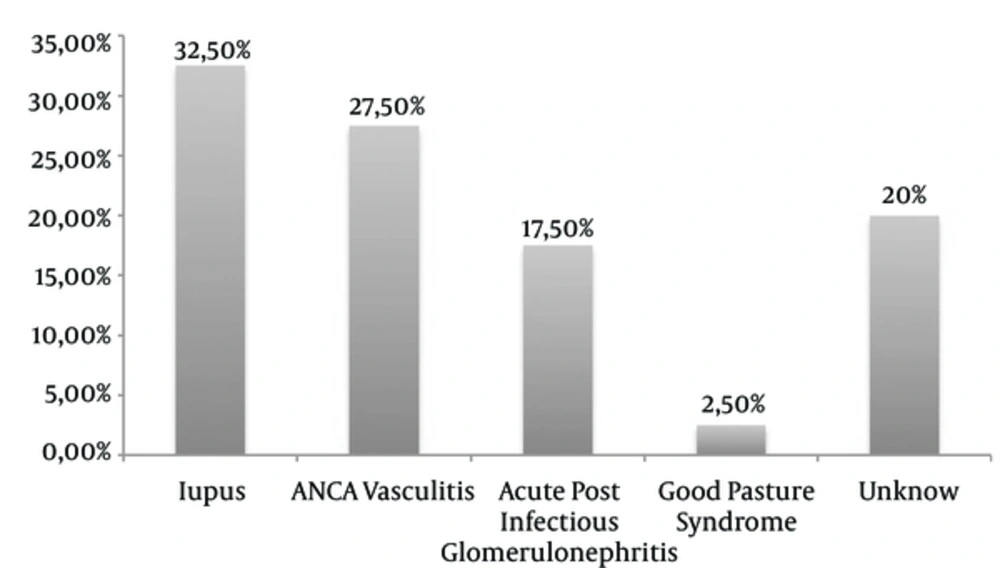
Lupus was the mean etiology found in 13 patients (32.5%), associated in one case with Gougerot-Sjögren syndrome and another case with scleroderma. ANCA-associated vasculitis was present in 11 patients (27.5%) with 4 cases of microscopic polyangiitis, 1 case of granulomatosis with polyangiitis and 1 case of polyangiitis with eosinophilia. Acute post-infectious Glomerulonephritis (GN) was found in 7 patients (17.5%): 2 had the otorhinolaryngological pathology, one had post-endocarditis and 2 were post-malaria cases. For the other 2 patients, the origin of the infection was not specified (Figure 1).
From a therapeutic point of view, hemodialysis was used in 50% of the patients. The mean number of sessions was 6.11 ± 0.93. Etiological treatment varied according to the cause, essentially relying on intensive immune-suppressing medication combining corticosteroids and cytotoxic drugs. Unfortunately, 10 patients could not continue treatment due to a lack of financial resources.
Regarding evolution, 7 (2.8%) patients were lost to follow-up. Among the remaining 32 patients, 6 patients (19.27%) had a favorable outcome with total recovery of renal function. However, in 26 patients (83.2%), the evolution was unfavorable. Twenty-three patients (73.6%) developed chronic renal failure and 3 (9.6%) died during hospitalization.
Regarding renal function, poor prognostic factors found in the current study with a statistically significant correlation with an unfavorable outcome were as follows, oligoanuria on admission (P = 0.028), high creatinine level on admission (P = 0.013), recourse to HD (P = 0.03), high percentage of fibro-cellular (P = 0.013) and fibrous crescents in the renal biopsy, and a high percentage glomerular sclerosis (P = 0.37). A high number of cellular crescents and lupus were correlated with a good renal prognosis (Table 2).
| Group B1 (n = 14) | Group A + B2 (n = 16) | P Value | |
|---|---|---|---|
| Gender ratio | 8M/6F | 2 M/14F | 0.010 |
| Oligoanuria at admission | 14 cases (100%) | 7 cases (43.75%) | 0.0008 |
| Mean blood creatinine at admission, mg/L | 91.54 ± 46.4 | 26.28 ± 17.95 | 0.0001 |
| Use of hemodialysis | 13 cases (92.86%) | 6 cases (37.5%) | 0.0001 |
| Cellular crescents | 66.67% | 20.83% | 0.028 |
| Fibrocellular and fibrous crescents | 33.33% | 83.33% | 0.013 |
| Scleroric glomeruli | 1.17 ± 0.98 | 4.24 ± 3.33 | 0.037 |
4. Discussion
The prevalence of crescentic glomerulonephritis is around 2% to 10% of all glomerulonephritis cases in most studies: found at a rate of 7.4% in Morocco and 8.2% in South Africa (4-6). In Germany (7), as in the current study, the prevalence was 5.33%. The annual incidence in France was 0.7/100,000 (8). In an Iranian study about histopathologic patterns of adult renal disease, Mardanpour found a prevalence of 4.5% of crescentic glomerulonephritis (9). The patients of the current study were young (mean age 33.9 years) with a female predominance (the male: female gender ratio was 0.53). These results are similar to those reported in the literature (6, 10-13). In the current study, the mean time from first symptoms to admission in a care unit was 40 ± 29.66 days. This time limit exceeded 15 days for 70% of patients. This could be explained by the fact that patients were treated in health centers or by traditional medicine before coming to the nephrology unit. Hypertension was found in 65% of cases, associated with oligoanuria in 62.5%. In Ozturk’s series, 21 patients (51.2%) had oliguria-anuria (14).
Upon admission, renal failure was found in 75% of patients, close to the value of 64% in the series published by Husseini (10), versus 90% in the Arrayhani series (4), and 92.4% in the series described by Zheng (6) (Table 3). However, Ozturk found that 22 patients (53.7%) were on dialysis because of acute clinical and laboratory indications (14). Histologically, crescents were cellular in 60.5% of cases. This was similar to Arrayhani’s results, showing cellular crescents in 61.7% of patients (4). However, Husseini found that 27% of patients had cellular crescents in his series (10). Crescents were fibrous in 32.5% of cases in the current study versus 11.7% in the Husseini’s series (10). The presence of fibrinoid necrotic lesions, cellularity and rupture of Bowman’s capsule leads to active inflammatory lesions (2). The percentages of completely sclerotic glomeruli (5.15%), interstitial fibrosis (20%), and tubular atrophy (50%) attest to the chronicity of these lesions. Lesions of different duration show a rapid evolution towards chronicity in the absence of early treatment. The importance of these tubular/interstitial lesions found in the current series could be explained by the excessive use of phytotherapy in 47% of patients; this aggravated the renal disease. The severity of these histological lesions found in the current series may explain why only 6 patients (19.27%) had a favorable outcome with total recovery of renal function.
| Current study (n = 40) | Arrayhani and Amraoui (4) (n = 68) | El-Husseini et al. (10) (n = 128) | Andrassy et al. (7) (n = 33) | Tang et al. (6) (n = 172) | Ozturk et al. (14) (n = 41) | |
|---|---|---|---|---|---|---|
| Clinical presentation | ||||||
| Hypertension | 65 | 76.5 | 68.4 | - | 60.4 | 46.3 |
| Oligoanuria | 62.5 | 44.6 | 48 | - | 50.6 | 51.2 |
| Renal insufficiency | 75 | 64 | 90 | - | 92.4 | 53.7 |
| Etiology | ||||||
| Lupus | 32.5 | 42.6 | 28 | 9 | 20.5 | 9.8 |
| ANCA vasculitis | 27.5 | 27.9 | 4.6 | 75.7 | - | 63 |
| Acute Post infectious glomerulonephritis | 17.5 | 17.6 | 17.9 | 0 | 28.7 | 4.9 |
| Evolution to ESRD | 48.8 |
aValues are expressed as %.
Regarding the etiologies, lupus was the first cause of ECGN found in the current series, with a percentage of 32.52%. This finding is similar to that of previous studies by Arrayhani and Amraoui (4), Husseini et al. (28%) (10), and Tang et al. (6) (20.5%), whereas it was found only in 9% of cases in Andrassy’s German series (7) and 9.8% of cases in Ozturk’s Iranian series (14). The high incidence of lupus in these African series could be explained by ethnic and racial factors. Indeed, the prevalence of lupus is more important in African-American populations than in Caucasian-American populations (8). According to an English study conducted in Nottingham, prevalence of lupus is 24.7/100 000 in the population as a whole against 207/100 000 for the black population of the same region (8). Vasculitis was responsible for ECGN in 75.7% of cases in the German series (7) and 63.4% in the Iranian series (14), whereas it was the cause of lesions in 27.5% of the patients of the current study, 27.9% in a Moroccan series (4) and 4.6% in an Egyptian series (14). Infections related to ECGN were found in 17.5% of cases for the current series, 17.6% for Arrayhani (4), 17.9% for Husseini (10) and 28.7% for Zent (15). This etiology was found in 4.9% of cases in Ozturk series (14) and not found in Andrassy’s work. The decrease in the incidence of post-infectious ECGN in Europe could be explained by improved standards of living and better medical care in Western countries compared to the developing world (Table 3).
On an evolutionary level, after an average follow-up of 7 months, 3 patients died. Of the remaining 30 patients, 18% had normal renal function while 53% had kidney failure. Forty-three percent (43%) of cases in the current study developed end-stage renal disease (ESRD), as in most other series except for Andrassy’s (7) where the progression was considerably better, with 55% recovering normal renal function. In Ozturk’s series, twenty patients developed end-stage renal disease (14). This could be due either to ethnic factors (Caucasian versus African and Asian), etiological factors (vasculitis being the dominant etiology in this European series) or socio-economic factors. The economic level allows better access to care, availability of treatment, and comprehensive care. The ECGN secondary to lupus seemed to have a better prognosis in the current series and in the Arrayhani series (4), unlike that of Zent et al. (15) where 87% of patients had either ESRD or were deceased. Of these risk factors, 3 were common in the 4 series: oligoanuria, high creatinine level on admission and a high percentage of fibrous crescents. The current research was consistent with the series by Arrayhani and Amraoui (4), showing a high percentage of sclerotic glomeruli.
4.1. Conclusions
In conclusion, ECGN is a cause of rapid and irreversible renal function impairment. It was relatively common in the current study. Etiologies were dominated by lupus and infections. The relatively long period of treatment delayed diagnosis and management. This was at the root of poor recovery of renal function in the current series. The risk factors identified in the current series could improve the prognosis of ECGN in the studied population.