1. Context
Leptospiras, a genus of spirochetes, is restricted to aerobic environments and consists of 2 species, including L. interrogans (also known as pathogenic form) and L. biflexa. They can lead to leptospirosis, which accounts for 873,000 infections and 48,000 deaths all over the world in one year. For this reason, it is considered a huge threat to human health (1). Many kinds of animals could preserve Leptospira, yet the rat is known as the major reservoir of the bacteria (2). Most commonly, human infection occurs after contact with water, soil or food that has been contaminated or through exposure to infected animal tissue/urine (1-3). This disease could occur in some occupations more than others, for instance, in farmers, trappers and rice field workers; this explains why the other name of this disease is “rice field fever” (1, 3).
Pulmonary, hepatic, and renal failure could occur due to either mild or severe leptospirosis, especially among paddy workers in East Asia. For this reason, one of the most important difficulties in this region is the occurrence of leptospirosis (4). Regarding the clinical presentation of leptospirosis, 85% to 90% of cases present fever in the absence of jaundice, which eventually subsides itself without any treatment. The other probable complications are lung bleeding accompanied by respiratory failure (2, 5) and 5% to 10% of cases manifest as inflammation of only meninges or both meninges and brain concomitantly (3).
The most serious condition in this setting is “Weil’s syndrome”, which encompasses signs, including fever in association with hemorrhagic tendency. The second sign is hepatic impairment, and the third is “ARF”. Without appropriate treatment for these signs, they can lead to death (1). Renal fibrosis is a complex process, in which a variety of boyh resident and infiltrating cell types are involved. It is considered as the most important mechanism of “CKD” in leptospirosis (4). Renal involvement is common due to extreme ability of this bacteria to attack renal cells, in addition to rich blood supply of the renal texture. Although T cells and B cells of the host are activated during exposure to leptospira Ag, they can’t defend appropriately because of the escape of this bacteria from immune response (6). The incidence of leptospirosis has been increasing in some endemic countries (2). In Thailand, for instance, the rate of disease has become 30 folds greater since 1995 to 2000 (7).
Leptospirosis incidence has been previously investigated in Iran and a cohort study was done in Rasht, one of the biggest cities of the north of Iran, and showed a number of patients were infected with these bacteria and eventually died from 2008 to 2010. This disease is endemic in this city because more than 10% of people are infected by these bacteria. Finally, in this cohort study, among 600 patients, 9 died (8). The reports from different parts of Iran have indicated the increasing incidence of the disease (9). This disease is not among critical catastrophes for the countries’ government, yet the importance of this disease is its high prevalence and death rate (10). The clinical manifestation of leptospirosis could be separated to 2 stages: The first stage continues for 3 to 7 days. Patients complain of high body temperature, serious headache, and chills. Loss of appetite, nausea, diarrhea, vomiting, malaise, and muscle pain come after the previous symptoms. Fever is more than 38 degrees centigrade and less than 39 and vanishes after 4 to 7 days from initial symptoms. In this first stage, leptospira can be confined to the blood. In approximately one-fifth of patients, the complaints of this stage disappear before the beginning of the immune phase, which continues for 4 to 30 days. In the second stage, more serious complications, such as inflammation of the meninges can occur. IgM antibodies are generally found at this stage and the amplitude of leptospirosis is related to the severity of the hosts’ humoral immune response to leptospira (2, 3, 5). In Taiwan, the most prevalent manifestation of this disease is febrile icterus in association with acute kidney injury (10). In Iran, one of the most common clinical symptoms of this infection in patients was ague (fever and chills) and the second symptom was jaundice at the time of admission. High total bilirubin level might also be a warning sign, especially oliguric renal failure, which may result in secondary toxic effects of bilirubin (11).
2. Renal Complications
2.1. Epidemiology
In developed countries, leptospirosis is a rare etiology of acute kidney injury. Nevertheless, in tropical countries, regardless of being either developed or non-developed, in addition to being one of most important etiologies of acute kidney injury, leptospirosis is endemic. The occurrence of acute kidney injury ranges from 10% to 60%, according to the severity of the disease and age, and denoting exactly what the AKI’s meaning is. In a number of countries like India, leptospirosis was reported in 31% of the ARF cases during 1987 to 1991, yet this percentage decreased to 7.5% during 1995 to 2004 (12). In both Thailand and Singapore, leptospirosis was found in more than 20% of AKI cases; 7374 cases of AKI have been found in the past 10 years in Sao Paulo, Brazil, of these patient, 6,777 patients were hospitalized at the “Hospital das Clinicas”, where only 60 patients (approximately 0.89%) had AKI due to leptospirosis (13). The percentage of ARF is one of the most common presentations of leptospirosis all over the world. Overall, 15% to 75% of patients with leptospirosis, experience this complication. Obviously this number increases in situations, in which a patient obtains this disease sporadically, or when the patient is very old or when the definition of ARF is extended to a very low increment in plasma creatinine level (13).
2.2. Clinical Manifestations
Renal complication in leptospirosis could range from a subclinical period, with mild proteinuria and atypical urinary sediment, to serious AKI. Both white blood cells (WBC) and red blood cells (RBC) could be found in the urine of these patients. If there is proteinuria in these patients, the amount is often lower than 1 g per day (to some extent this condition is considered as micro albuminuria in nephrology). Some other byproducts, such as biliary pigments and granular casts, can be present in the U/A of patients. In AKI of this disease, rapid increment in both serum urea and serum creatinine, could lead to “Icterus”. It should be noted that in kidney injury of these patients, hyperbillirubinemia is associated with a serious form of disease and usually correlates with either inability to produce urea, named “Anuria” or inability to produce sufficient urea, named “Oliguria” (14). Complications of the kidney could be observed in both the host and some carriers, such as dogs and other mammals (15, 16) (Figure 1).
Among 60 patients, 7.7% reported swimming in the past (n = 5), 85% were male (n = 55) and 58% (n = 36) were farmers. The most common complication of the disease was febrile icterus. The most widespread clinical manifestation of this disease was fever (88%), muscle pain (74%), and sweating (68%). Decrease in plasma sodium was seen in 7.5% of patients and its counterpart (hypernatremia) was seen in 72.6%. Two patients (3% of them) had hypokalemia. Thrombocytopenia was observed in 90% and leukocytosis was seen in half of the cases. Increment in the plasma level of both hepatic enzyme and bilirubin was seen in the majority of patients. For instance, “AST” increased in 95% of patients and bilirubin increased in all patients. Jaundice in association with bleeding in this disease was observed in 42.4% of patients within the follow-up period and 25% of them had ARF, 22% had pneumonia, 1% of had subarachnoid hemorrhage in the background of this “icterohemorrhagic leptospirosis”. Lastly, the fate of the patients was as follows: 38 patients (60%) left the hospital with residual impression,. Positive serology was observed in 75% and finally 4% died (17). In a recent survey, 58 patients were studied according to other organ complications, in which the most common involvement was “hemorrhagic diathesis” (80% of cases) and the least common was “rhabdomyolysis” (5% of cases). The other organ complications were insufficiency of liver function (72%), respiratory impairment (38%), and circulatory abnormalities (33%) (2). In another study in Iran, 51 patients with ARF due to severe disease were treated at an Iranian hospital between January 1st, 2007 and December 31st, 2012; incidence of renal complication was recorded as 7.6%. Most patients were males (82.4%) and the mean age was 53.5 ± 14.8 (22 to 79) years. Most of the patients were admitted during summer (rice cultivation season). The predominant symptom was fever, although the length varied between 1 and 21 days, followed by nausea and vomiting, muscle pain and jaundice. Leukocytosis (> 10.000/mL) was common in 47%. Thrombocytopenia (< 150.000/mL) occurred in 28 (55%) patients (11). For the first time in Iran, a detailed study according to surface epithelial cell was done in Mazandaran from clinical data available for 119 suspected cases.
Among the 119 cases, 70 (59%) were admitted to the hospital and only 40 of them (55%) had the diagnostic criteria for confirming the disease by the means of specific molecular method. Overall, 73% of cases were male and the most common complication was the febrile condition in association with sweating and in some cases, icterus. The absolute treatment of patients was antibiotics (18). Among 840 in-hospital cases of leptospirosis in Brazil, approximately one-seventh of the patients in the mentioned study were younger than 18. Pain of the head and muscles in association with the febrile condition were the most common complications. In the mentioned study, patients, who were admitted to the hospital, were discharged 5 days later. Most of the patients were males, who had icterus and renal impairment in the background of this disease (19).
Another survey done in Thailand on 148 patients, who had leptospirosis, investigated the correlation of renal and pulmonary complications with hypotension. This study compared patients of different types of renal complications. Their first variant was normal renal function. The second was prerenal ARF (31% of the patients), and the third was ARF, which didn’t respond well to hydration (26% of the cases). It should be noted that this categorization is based on the probability of acquiring either hypotension or pulmonary failure. Accordingly, patients with normal renal function were investigated in this article. In this study patients of the so called group (normal renal function group) acquired hypotension with the probability of 48%, in spite of not receiving any pulmonary complication. As mentioned above, another category, which was examined in this study, was patients with pre renal “ARF”. The recent group obtained hypotension with a probability of 65%, and pulmonary complication with a probability of 6.5%. Lastly, the third group (ARF which didn’t respond well to hydration) was investigated. Among patients in the recent group, 74% developed pulmonary complications and 87% experienced hypotension (20). A cohort study was done in Brazil, in which 195 cases with the combination of different ages from 15 to 90 were studied. Overall, 80% of participants were males and most of them were farmers, office workers, and garbage collectors, and totally accounted for 50% of cases. As noted above, the most common complication was febrile icterus state (93%) in association with muscle pain and headache (82%), and sweating (60%). Inability to produce enough amounts of urea was observed in 29% of patients. Tendency of bleeding was seen in 33% of patients. This complication comprised of purpura (19%), hemoptysis (11%), and some upper and lower gastrointestinal bleeding. Overall, 82% of patients had “acute renal failure” in association with hypokalemia. The ratio of BUN/Cr increased due to different mechanisms of pre-renal and renal failure (21). Involvement of the kidney due to leptospirosis is non-oliguric and has better prognosis in comparison with its counterpart, oliguric kidney injury. One of the other water-electrolyte disturbances in this disease is “hypokalemia”, which is on the contrary with other types of renal failure, which could lead to “hyperkalemia”. This hypokalemia could be seen in approximately 45% of involved patients (22, 23). In non-oliguric cases, creatinine is usually less than 4 mg/dL (24). Oliguria and anuria in severe cases are usually accompanied by dehydration, jaundice, and severe sepsis. Coexistent hematuria and rhabdomyolysis is a risk factor for disease progression. Therefore, renal failure can be a consequence of direct effect of leptospirosis along with jaundice, dehydration, and rhabdomyolysis (23, 25). Hypokalemia’s report due to leptospirosis could range from 45% to 74% in patients admitted to the hospital and this could lead to intravenous potassium replacement in 80% of cases (26). Hypovolemia could induce aldosterone and cortisol increases despite hypokalemia (27).
3. Pathogenesis
Entering the texture of the body by the means of perforating its barriers is the first step of pathogenesis. The second mechanism is spreading through the blood system to arrive to its target. In this leptospiremic state of the disease, the bacteria could be detected by the means of the polymerase chain reaction (PCR), in which the presence of 106 bacteria in each milliliter of blood is diagnostic. Although this level resembles the diagnostic level of “Borrelia” in the Lyme disease, it differs from this level for diagnosing Escherichia coli and the rest of Enterobacteriace. Another difference between the response to E. coli and Leptospira is dissimilarity between the sensitivity to the response of these 2 bacteria. This means that low level of lipopolysaccharide in E. coli is enough to activate the immune system, and on the contrary with the response to “Leptospiras’ antigen”, which activates the immune system at a higher level. It should be noted that the other mammals’ TLR4 could differentiate LPS of leptospira as well as the Enterobacteriace. According to other types of infections, accidental hosts in this disease could encounter a catastrophe on the contrary with the natural host of the disease. The former state in this disease occurs in humans and the latter state could be seen in mice (3, 28). The “OMP” gene of leptospira is responsible for hurting the surface epithelial cells directly. Although many proteins could be observed in the cultivation of this bacteria, only a few of them can be seen in the process of pathogenesis. The protein of the outer membrane of leptospira could lead to injury of the host’s cell by the means of complement activation. The tubular injury due to leptospirosis could originate from the “OMP” gene of the leptospira, such as “LipL32”, which can result in accentuation of the cascades dependent on hosts’ toll-like receptor, and can result in the activation of all NF kb, kinases, and cytokines (2). The “KDO” component of the “LPS” of this bacteria could be detected by TLR2 of both human and animal counterpart. Interleykin-6 (IL-6) and Tumor Necrosis Factor (TNF)-alpha rises in this disease and this increment is the leading cause of symptoms. Whenever these increments are extremely high, the patient experiences a state named “cytokine storm”, in which the patient suffers a lot from his or her disease. There is a wide variety of renal complications ranging from non-oliguric to oliguric renal failure . Due to impairment of Na/H exchanger in the proximal tubule in the “Weil’s syndrome”, polyuria in association with renal failure, is a characteristic clue for diagnosing the so-called syndrome in the background of this disease (29). The powerful pathogenic mechanism of leptospira in inducing AKI is the first direct nephrotoxic activity of the leptospira, the second is the immune response of the host, which is triggered by the toxin of the leptospira, and the third is both hypotension and hypovolemia. Lastly, there were some other mechanisms for the genesis of AKI in leptospirosis. These include hemodynamic change, icterus, and rhabdomyolysis (30) AKI due to AIN in the background of leptospirosis, and has been shown in some experimental studies to be correlated with the presence of leptospira in the texture of the kidney. Furthermore, AIN occurs after tubular damage (30). Two distinct histologic alterations could be considered in the setting of this disease. These 2 alterations are named “tubulointerstitial disorder”, in which dilatation of tubular cavity in the expense of precipitating hyaline casts in addition to the atrophy of the surface texture of the tubule could be seen (31).
The period during which the patients have had leptospirosis, is correlated with the type of both kidney complication and edema. This means that if patients had both ATN and cell edema concomitantly, they would die within the first week of the disease. Another variant could be considered the condition, in which patients had ATN and interstitial edema (not cellular edema) and died 2 to 3 weeks later. The last variant of patients in this survey was patients, who had severe and diffuse interstitial nephritis, and died after 3 weeks (2, 30). Entrance and differentiation of leptospira mainly occurs in the cavity of the tubule. Main Ag of the bacteria, such as” LipL32”, could be recognized by the surface cell of the tubule. Afterwards, some mechanisms occur and will lead to migration of monocyte to the involved area. Then, the other type of inflammatory cells, such as plasma cells and lymphocyte, can infiltrate to the area and result in aggravation of inflammation and edema (32). One interesting outcome of some experimental studies, is that tubular dysfunction due to leptospirosis could occur whether kidney injury is present or absent. As mentioned earlier, one of the good prognostic factors in AKI due to leptospirosis, is non-oliguric variant of AKI. It is well-known that most forms of ARF are associated with hyperkalemia. Therefore, one of the distinctions of AKI due to leptospirosis with other types of AKI, such as AKI due to malaria, diphtheria, and meningococcemia is that AKI due to leptospirosis both increases the plasma potassium level and decreases the plasma potassium level (2). The fate of renal complication in leptospirosis ranges from intact tubuloglumrolar function to fibrosis of interstitium and segmental glomerulosclerosis, which is seen in highly advanced patients (32).
4. Leptospira Therapy
The most critical step in curing this disease is immediate recognition and injecting appropriate drugs as soon as possible, followed by suitable treatment. There are different antibiotics in treatment of this disease, according to the time of their developmental diagram. Doxycycline is effective at both the stationary and growth phase. Gentamycin’s role is similar to doxycycline. Cefotaxime and azithromycin are effective in the exponential phase exclusively. There is no agreement about the usage of antibiotics for curing leptospirosis (33-35). Although treatment with “IV penicillin” has been implemented for a longer duration than other therapies like ceftriaxone, the latter is much more effective than the former nowadays, according to a prospective cohort study (36). Antibiotics, if started at an appropriate time, can lessen the duration of febrile state and renal impairment and the time of admission to the hospital in comparison with patients, who do not receive these medications.
A 7-day therapy with antibiotics is prescribed for the treatment of leptospirosis. The form of antibiotic is different, according to the severity of the disease. For instance, oral forms of antibiotics, such as tetracycline, farmentin, and gentamycin could be prescribed in uncomplicated cases. However, these oral forms aren’t effective in severe cases, according to the recommendation of the world health organization (WHO) in 2003. Therefore, penicillin (IV, 1,500,000 unit) (4 times a day), ceftriaxone (1 g once a day) or cefotaxim (1 g four times a day) should be prescribed for severe cases of leptospirosis. However, antimicrobial therapy is most effective in less severe cases yet they do not work as well in complicated cases. For this reason supportive therapy is the goal of treatment in the management of a patient with complications, such as renal impairment (37, 38). Because of the importance of these bacteria in endemic areas, people should be protected by prescribing doxycycline. Penicillin like any other drugs has many adverse effects. Among these, Jarisch-Herxheimer reaction occurs in this disease, which is correlated with the induction of cytokines’ release and production of toxins that are released from bacteria during their lysis by antibiotics. The antibiotic therapy is not stopped Jarisch-Herxheimer reaction occurs (39, 40).
5. Treatment – Supportive Therapy and Dialysis
AKI can originate from hypovolemia, which is the most important fertile soil for AKI and is present in most patients. Although it is important to hydrate these kinds of patients (patients with hypervolemia) orally, it should be avoided to compensate the whole loss of volume right away. This means that careful administration of intravenous salin for preventing hypervolemia and pulmonary complications is essential (2).
A nonoliguric form of ARF could be achieved after injecting lasix to the patients, who formerly had the serious oliguric form. There are some supplementary medications, such as dialysis, which is used alongside the main treatment. Although peritoneal dialysis is commonly used, its counterpart (hemodialysis) is more powerful for managing the patients, who concomitantly have acute kidney injury and bacteremia, according to a cohort study done in Thailand (30, 41). According to a recent case study of patients with AKI due to leptospirosis, 2 treatment methods were compared, including hemodialysis and peritoneal dialysis. Hemodialysis can better resolve the complication of leptospirosis, such as high bilirubin level, high plasma urea and cratinin level, than its counterpart, standard peritoneal dialysis (42).
Dialysis through the blood vessels is now the treatment of choice for complicated cases of AKI. This is in contrast with usual AKI cases in leptospirosis, for which dialysis through the peritoneum is the treatment of choice. All of this data was extracted from a large cohort study done in Brazil during the 1990s. This study was continued until the 2000’s decade and examined whether daily dialysis through the blood vessel is as effective as the last method for these kinds of patients. This newer study showed a decrement of 9% in the mortality of these patients during this newer span (2000’s decade). Although daily dialysis through blood vessels is more effective than its counterpart, dialysis through peritoneum is more acceptable for patients (21). There is no agreement about the most effective dialysis method for curing leptospirosis. As a result, all of the existing methods of dialysis, encompassing hemodialysis/peritoneal dialysis/hemoperfusion, have already been used (13).
6. Amelioration of Renal Function
In the form of AKI with lack of jaundice, after a few days or one week, renal function ameliorates by itself. Correction of some complications can occur at the same time. For instance, reduction in urea, creatinine, and bilirubin level alongside with the increment in the number of platelets could be seen within the second week of the disease (5). In a cohort study of 35 patients with leptospirosis, ARF, GFR, and urinary acidification, both the ability of proximal collecting tubule to reabsorb sodium normally and the ability of the kidney to not excrete protein in the urea were normal, whereas decrement of the urinary concentration until the end of the follow up, in 6 months was seen (30, 43).
According to a cohort study done in Finland on 53 patients admitted to the hospital, 60% had appropriate renal function after 2 months, 15% didn’t have any significant renal complication initially, and 25 didn’t get better until the end of the follow-up. Renal function did not correlate directly with the state of blood pressure in younger patients with “Leptospirosis”, because renal problems in most of the patients resolved after 2 months yet in one case hypertension was not eliminated (44).
If there are no other complications except for AKI due to leptospirosis, (such as pulmonary difficulties, increased level of both bilirubin and potassium level in plasma, oliguria, anuria, diarrhea, old age and either accompanied infection or background disease, which aggravate the prognosis), a suitable prognosis for this disease could be considered. If there are complications, the case fatality rate in leptospirosis would range from 12% to 36% (2, 45, 46).
7. Mortality
Patients, who are infected with larger size of Leptospira’s bacterium are more prone to encounter many complications until the end of the disease. The most pathogenic size of these bacteria is 15,000 bacteria in each milliliter of blood. Truculo showed this relationship for the first time (47).
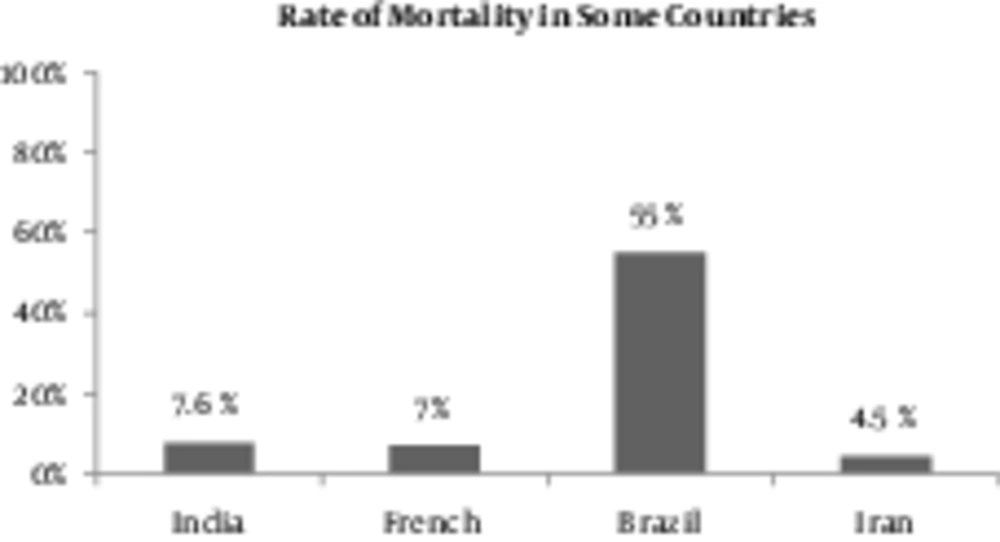
Some surveys in different countries, such as Brazil, Thailand, and Turkey, have been done for detecting the prognostic parameters which effect leptospirosis evolvement by using logistic regression. It has been proposed by this study that case fatality rate of this disease can range from 15% to 18%. It seems that AKI is responsible for most of the mortality in leptospirosis and without its presence, death from leptospirosis is unusual. It should be emphasized that “lung” and “kidney” difficulties were independent factors correlating with mortality. It has been reported that changing mental status, WBC count, platelets’ count, and alteration in ECG could be seen. Age has been proposed to have an indirect effect on prognosis in leptospirosis. In one study from Iran, 66 patients were investigated, 20 of which developed ARF and mortality rate was 6.1% (17). Within the epidemic period of this disease in India, 7% of patients eventually died, yet this number was approximately 6% in France (48). In Brazil, 42 patients, who had pulmonary hemorrhage were analyzed, from which 55% mortality rate was reported for 66% of patients, who had pulmonary hemorrhage and AKI concomitantly (46).
In another cohort study done in Brazil on 44 cases younger than 15, 75% developed renal complications and 7% had pulmonary complications and 2 death occurred among them (49). Some mortality risk factors in leptospirosis were reported through a retrospective cohort study done in Brazil with 110 patients, including oliguria, cardiac arrhythmia, difficult respiration and pulmonary complications (12, 49, 50).