1. Background
Renal colic, due to ureteric calculus, is a recurrent common clinical entity among those who attended the emergency department (1, 2). This pain is one of the most severe pains ever known to man. Mechanisms suspected of inducing renal colic includes: spasm of smooth muscles, increased peristalsis, increased pressure and inflammation at level of calculus (3-7). The definite treatment to relieve renal colic is to relieve the obstruction made by calculus using a stent, however, as far as most of the obstructions are partial, a medical approach is chosen (8, 9).
Medical approaches to renal colic mainly aim at relieving one of the mentioned mechanisms leading to renal colic. Opioids and non-steroidal anti-inflammatory drugs (NSAIDs) are the mainstay of renal colic treatment, however, side effects due to these medications are common (10, 11). Side effects of opioids are nausea, vomiting, constipation, respiratory depression, and dependence (12). Although NSAIDs are known as effective analgesics, in renal colic due to calculus NSAIDs interfere with renal auto-regulatory response to obstruction, which is tolerated in healthy individuals, however, it may lead to renal failure among high-risk patients with coexisting renal disease (13, 14). Considering these side effects, researchers have focused on proposing new medications not only to increase effectiveness, but also to decrease the side effects. Paracetomol is a common well-known analgesic and anti-pyretic agent, it comes in oral, rectal, and intravenous formulations (15-19). Intravenous paracetamol have an onset of effect of less than 10 minutes, which reaches its peak in 15 minutes (15); an analgesic with this feature can be presented as a new medication for renal colic with less side effects. Effectiveness of paracetamol as an analgesic has been proven in dental surgeries, orthopedic procedures and lower back pain (18, 20, 21).
In the present study, in order to offer paracetamol as an effective alternative for routine medication of renal colics, efficacy of morphine plus diclofenac as a typical medication used worldwide for renal colic was assessed then it was compared to analgesic effects of paracetamol among patients with renal colic.
2. Methods
2.1. Study Design and Settings
In this single-center, prospective double-blind randomized clinical trial was performed in the emergency department (ED) of Emam Reza clinical-educational center, Tabriz- Iran, which is the referral center of northwest Iran from June 2014 to January 2015.
2.2. Selection of Participants
Considering renal colic incidence ratio of 29.3%, power of 0.2, confidence interval of 0.05, and loss probability of 20%, a sample size of 80 was calculated throughout the study. Then, to increase the power of the study, 90 patients were designated as the population for Intervention and control group. Therefore, in this study 180 patients attending ED from 18 - 65 years old and flank pain accompanying documented urinary system stone based on imaging methods (CT scan and ultrasonography) were randomly included in study. In case of any refusal to give informed consent, hemodynamically unstable, history of renal failure, allergy to morphine or paracetamol contraindication to NSAID usage, and history of analgesic administration in past 6 hours of patients were excluded from study. Although treating physician’s varied, the chief resident who was familiar with the protocol was consulted to decide on the patient’s eligibility.
2.3. Interventions
Included patients were blindly and randomly divided into two equal groups of intervention (group A) and control group (group B). Patients in Group A were administered with a single 1 gram intravenous dose of paracetamol (Apotel 1 gr, Unipharma, Attica, Greece) solved in 100cc of normal saline. Patients in group B were administered with 0.1 mg/kg of morphine plus 75 mg of diclofenac (Dicloflame, Unipharma, Attica, Greece) solved in 100 cc of normal saline. All solutions were administered in 10 minutes. One of residents blinded to the study provided the randomization table. One of the nurses prepared the medications and another nurse infused solution to patients, which they were not aware of the content prepared by the nurses.
2.4. Variables and Measurements
The first variable was demographic information and position of renal calculus, which were recorded in the checklist. The main variable measured in the present study was pain severity, which was assessed 0, 10, 20, 30 minutes, and 24 hours after medication was administered using visual analog scale (VAS). For better cooperation, VAS system for pain measurement was explained to the patient and all data was collected by one individual. Side effect due to medications, such as mentioned by the patients including nausea, vomiting, dizziness, vertigo, dizziness, palpitation, and diaphoresis were recorded in the checklist.
2.5. Statistical Analysis
Normality analysis was performed using the Kolmogorove Smirnov test and all obtained data was presented as mean ± standard deviation (mean ± SD), distribution, and percentage (%). Chi-square test was used to compare quantitative variables. U Mann-Whitney test was used to compare the intervention and control group. The used statistical program is SPSSTM Version 15 (SPSS ltd, Chicago, IL, USA). P value less than 0.05 was considered statistically significant.
2.6. Ethical Considerations
In the present study no intervention was applied by the researcher on patients out of the required area and no extra expenditure was imposed to included patients. However, patients were free to quit the study, during any level of the study, if they wanted. All participants had signed a written consent. The study protocol was approved by the ethics committee of Tabriz University of Medical Sciences (TUMS), which is in compliance with Helsinki declaration. In addition, this study is registered in Iranian registry of clinical trials (IRCT), with code of IRCT201405127327N3.
3. Results
Of all patients, in the intervention group (N = 90) the mean age was 40.86 ± 15.12 years old and 55 patients (61.11%) were male, while in the control group (N = 90) the mean age was 38.57 ± 12.18 years old and 52 patients (57.77%) were male; there was no statistically significant difference between groups considering age (P = 0.26) and gender (P = 0.76). In the intervention group, 51 patients (56.66%) had calculus in the left ureter or left kidney, while in the control group, 49 patients (54.44%) had calculus in left ureter or left kidney; this difference between these groups was not statistically significant (P = 0.88).
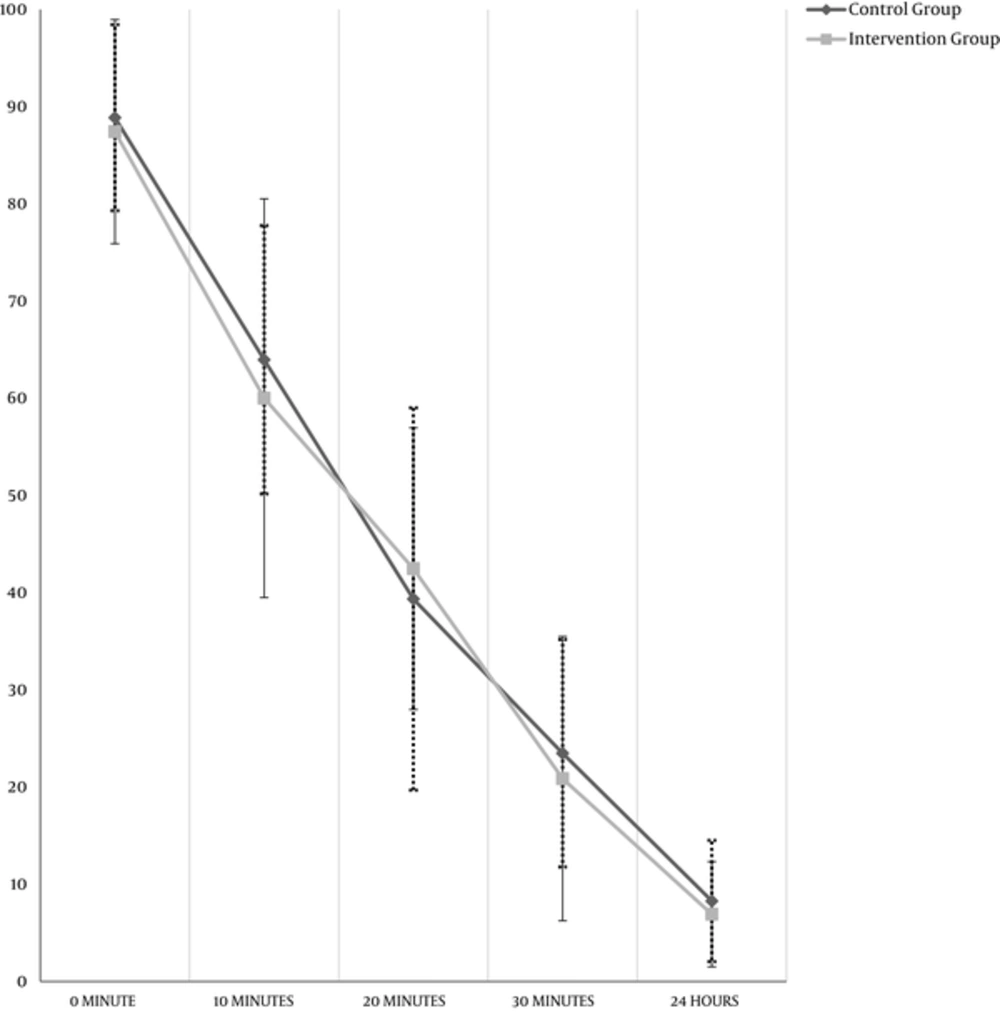
Patient’s pain severity based on VAS on 0, 10, 20, 30 minutes, and 24 hours after administration of medications were obtained (Table 1); there was no statistically significant difference between the groups. Based on the VAS score, pain reduction, which is shown in Figure 1, in both groups, the VAS score decreased statistically significant from one-time stage to another (P < 0.05).
Considering side effects, nausea was the only side effect that occurred, which affected 14 patients (15.55%) in group B, while no side effects were recorded for group A; thus, there was a statistically significant difference considering side effects between group A and group B.
| Time Stages | Intervention Group | Control Group | P Value |
|---|---|---|---|
| 0, min | 87.43 ± 11.53 | 88.87 ± 9.57 | 0.36 |
| 10, min | 60.01 ± 20.51 | 63.96 ± 13.80 | 0.13 |
| 20, min | 42.47 ± 14.49 | 39.35 ± 19.67 | 0.22 |
| 30, min | 20.87 ± 14.62 | 23.48 ± 11.70 | 0.18 |
| 24, h | 6.92 ± 5.41 | 8.28 ± 6.24 | 0.12 |
aData is presented as mean ± SD, millimeters.
4. Discussion
Renal colic is one of the most common diagnoses made in ED, which considering its severity, needs medical or surgical approach. As far as renal colic are mostly due to partial obstructions, which requires medical approach to decrease pain (3, 4). The mainstay of the current medical approach are analgesics, which is composed of an opioid and a NSAID (9). In this routine, treatment opioids cause an early-onset pain reduction while NSAIDs have a more prolonged effect. Analgesics selection in different medical conditions are made based on analgesics effectiveness while trying to decrease side effects (22, 23). Paracetamol is known as an effective analgesic with minimal side effects; therefore, in the current study effectiveness of paracetamol vas compared with morphine plus diclofenac is a routine medication for renal colics. Based on the current study, there was no statistical difference between paracetamol and morphine plus diclofenac in decreasing pain, in addition, considering the side effects, paracetamol had statistically significant less side effects.
Based on the literature intravenous paracetamol is mostly used in settings of post-operative pain management (20, 24-27). In a study by Moller et al., it was tried to compare the analgesic efficacy and safety of a single intravenouse infusion of 1 gram of acetaminophen with propacetamol 2 gram (a pro drug form of paracetamol) and placebo in patients with moderate-to-severe pain after third molar surgery. It was concluded that pain reduction in the intervention group was statistically more than the control group (26); the present study is similar to this study considering the final result, however, in this study placebo was solely used in the control group and not an analgesic.
On the other hand some studies have compared paracetamol with other medications in renal colic. In a study by Serinken et al., effectiveness of intravenous paracetamol versus morphine for renal colic was investigated and it was concluded that intravenous paracetamol effectiveness, as an analgesic, was similar to morphine while bearing less side effects (28); the results of this study are similar to the present study and the only difference between these studies is administration of diclofenac beside morphine in control group, while Serinken et al., only used morphine in control group. In another study by Grissa et al., comparing effectiveness of intravenous paracetamol and piroxicam in renal colic, it was concluded that paracetamol was statistically more effective than piroxicam (29); results of this study was similar to present study.
In the present study no side effects were seen in group A, while in group B nausea was the only and most common side effect recorded. Based on the literature, intravenous paracetamol is well-tolerated in clinical trials and incidence of side effects is very low (less than 1 in 10000) (30). In a study by Bektas comparing intravenous paracetamol and morphine for the treatment of renal colic, it was concluded that there was no statistically significant difference in side effects between morphine and paracetamol (31); this study was in contrast with the present study.
The main limitation of the present study is the limited population of study; therefore, conducting clinical trials with more study population may lead to a strong conclusion about the effectiveness of paracetamol versus routine medications. In the present study, just short-term and early-onset side effects were inspected, however, long-term and late onset side effects were not, therefore, designing clinical trials with longer periods of follow up may enrich the conclusion of the study.
4.1. Conclusion
Based on the present study, intravenous paracetamol can be proposed as an effective analgesic and comparable with routine medications for renal colic, especially when patients have a contraindication for NSAIDs or opioids. In addition, less side effects of intravenous paracetamol may propose this medication as a safer medication when compared to opioids and NSAIDs. Designing clinical trials with more study populations and longer period of follow up may lead to a more precise conclusion, therefore, intravenous paracetamol may be replaced with routine medications.