1. Background
Chronic kidney disease (CKD) is an important health concern in the world (1). Two causes of CKD are hypertension and diabetes (2). If the condition progress, the patients with CKD are at high risk of end-stage renal disease (ESRD) and they need dialysis or kidney transplantation (3). Reduced function of the reproductive system is often associated with ESRD (4). Many patients who suffer from ESRD experience sexual dysfunction leading to infertility, which is due to vasomotor dysfunction, endocrine aberrations, medications, and psychological factors (5).
CKD may affect the hypothalamic -pituitary-testicular axis and consequently, which resulted in impairment of spermatogenesis and spermiogenesis due to low levels of testosterone (6). During spermiogenesis, sperm chromatin is entirely restructured and undergoes characteristic rearrangements with vital compaction (7). The condensation of chromatin involves a replacement of testis-specific nucleoproteins, including histones by more basic proteins named protamines (8). These protamines pack nuclear DNA tightly into highly condensed chromatin; accordingly, the sperm nucleus acquires an important mechanical and chemical stability (9). The nuclear compaction is involved in the protection of the paternal genome during the transit of spermatozoa through the male and female genital tracts and during interaction with oocyte. Abnormalities in chromatin condensation can cause nuclear damages as DNA denaturation or fragmentation, which often are associated with male infertility (10). In addition, sperms with abnormal chromatin condensation could be a predictive factor in assessing the chances of fertilization and pregnancy (11).
According to conventional semen analysis,sperm with proper morphology, counts, and motility, is not homogenous populations. The main concern of modern andrology is Selecting competent of sperm (12). There are several techniques that could detect the abnormalities of sperm head, such as cytochemical assays (Toluidine blue and Aniline blue), flow cytometeric-based sperm chromatin structure assay, comet assay, and TUNEL assay (13). Toluidine blue (TB) is an acidophilic dye and stains sulfates groups in DNA, thus it is a simple and sensitive test for detection of damaged DNA structure (14, 15). Another dye is Aniline blue (AB) that stains lysine group in histones of the sperm DNA and it shows sperm chromatin condensation (16, 17).
2. Objectives
Since the CKD men have low volumes of semen and low or complete azoospermia with low motility and they have a high incidence of DNA damage (5, 8), Therefore, this study aimed to evaluate the sperm chromatin maturity of the patients with CKD before and after kidney transplantation. To achieve this aim, we used AB and TB for staining of sperm chromatin.
3. Methods
This study was a Quasi-Experimental study and carried out on 15 male patients who had chronic renal failure and were candidates for kidney transplantation that referred to Imam Khomeini Hospital of Ahvaz in Khuzestan province. The patients’ age was ranged from 16 to 65 years old. Transplanted kidneys were taken from alive donors. Patients with malignant tumors, heart failure, chronic respiratory failure, progressive hepatic disorders, diffuse vascular disorders, congenital urinary disorders such as exstrophy of the bladder, positive HIV viral infection, coagulative disorders, alcoholism, and other addictions were excluded from this study.
3.1. Ethics
The patients signed written informed consent for participation in this study.
3.2. Collection of Semen Samples
Three days before renal transplantation, the semen of patients was collected by masturbation and compared with another sample that was collected three months after the operation.
3.3. Semen Analysis
Semen samples allowed to liquefy for 30 - 60 minutes at 37°C. All parameters of semen (volume, concentration, motility, and morphology) were analyzed according to the World Health Organization (WHO) guidelines (18).
3.4. Toluidine Blue (TB) Staining
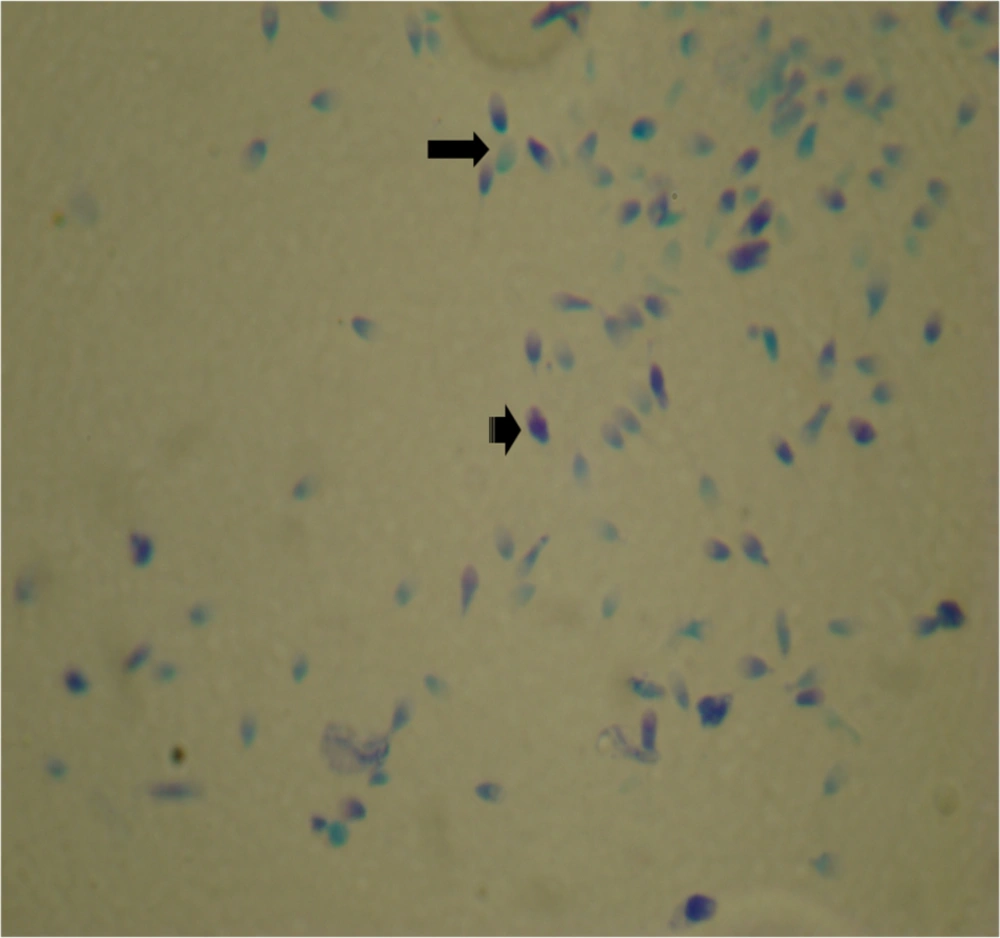
Sperms were pelleted at 1000 rpm for 10 minutes. Then 20 μL of sperm pellet was spread on glass slide and air-dried for 30 - 60 minutes. The smears were fixed with 96% ethanol-acetone (1:1) for 1 hour at 4°C; after that, they were hydrolyzed with 0.1 N HCl at 4°C for 5 minutes. The slides were rinsed 3 times and stained with 0.05% TB (in 50% McIlvaine’s citrate phosphate buffer, pH 3.5, Merck) for 5 minutes at room temperature (19).
3.5. Aniline Blue (AB) Staining
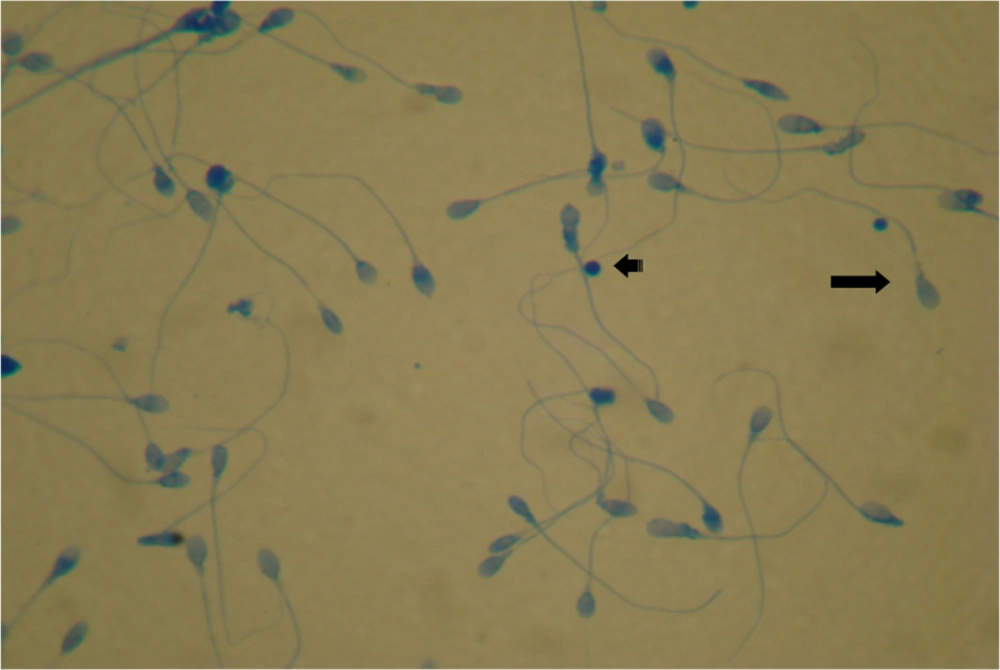
AB staining was performed as previously described by Hofmann and Hilscher (20). One mL of liquefied semen was washed twice with phosphate buffered saline (pH = 7.2) and 20 μL of sperm pellet was spread on glass slides. The Smears were fixed with 3% buffered glutaraldehyde for 30 minutes. The slides were stained with 5% aniline blue solution mixed with 4% acetic acid (pH = 3.5) for 5 minutes.
3.6. Grouping of Sperm Stained Heads
For both TB and AB stained smear, 200 spermatozoa in different areas of each slide were evaluated with a light microscope (100×). In AB staining, light blue sperm heads were defined as normal chromatin and dark blue sperm heads considered immature chromatin and damaged DNA.
In TB staining, due to the severity of staining, 3 grades were defined for sperm heads:
S0: Light blue as normal chromatin.
S1: Moderately blue as partial denaturation of chromatin.
S2: Severe dark blue as damaged DNA.
Light sperm cell head was scored S0 as negative staining that possessing DNA of normal and mature chromatin, and S1 + S2 (dark blue or purple) were scored as positive staining with damaged DNA and immature chromatin (Figures 1 and 2). The results were expressed as percentages of sperm stained heads.
3.7. Statistical Analysis
The analysis was performed by SPSS 22 software.
Data of study were expressed as mean ± standard deviation. Student's t test and Wilcoxon test were used to analyze parametric and non-parametric variables and a P value ≤ 0.05 was considered statistically significant.
4. Results
The mean age of the patients was 33.1. Thirteen patients (86.6%) were married and two (13.4%) were single.
Between married patients, six of them (46.15%) had a history of child abortion or infertility.
The mean values ± SD of semen parameters were summarized in Table 1.
Mean ± SD of Semen Parameters Before and After Kidney Transplantation in 15 Patients
The analyzed data showed a significant relationship between sperm count (P = 0.03), volume (P = 0.04), sperm motility (P = 0.007) of the patients before and after kidney transplantation; however, there was no statistically significant correlation in morphology parameter (P = 0.6).
The mean ± SD of positive TB staining (S1 + S2) was 45% ± 15.06 before the operation and this rate became 35% ± 7.59 with statistically significance (P = 0.01) three month after the operation. We also found significant and positive correlations between positive AB staining of sperm heads before and after the operation (40.64 ± 17.01 and 29.71 ± 15.83, respectively, P = 0.04) (Table 2).
Mean ± SD of Sperm Stained Heads Before and After Kidney Transplantation in 15 Patients
5. Discussion
The role of kidney transplantation is still controversial for maintaining normal sexual function (21). A study by Yu et al. showed that renal transplantation could improve spermatogenesis in uremic patients (22). The results of another study on rats with chronic renal failure who received bromocryptine plus to erythropoietin showed that these drugs could protect sperm DNA from oxidative damage and improve reproductive function (23).
A clinical trial by Akbari et al. was done on renal transplant patients. They reported highly significant changes in sperm motility and hormonal level; however, sperm morphology and concentration did not improve after renal transplantation (24). In another study Inci et al. found that in patients with uremia before or during adolescence, semen parameters were severely affected and spermatogenesis did not improve after kidney transplantation (25).
The results of our study were in agreement with McKay and Josephson findings, which evaluated the level of sexual hormones, spermatogenesis, and semen parameters before and after renal transplant (26). They reported that after transplantation, the hormonal disturbance would be improved, sperm count was increased while the rate of abortion was decreased. Similarly, in our study, the sperm count was significantly elevated after kidney transplantation.
In another study, Cao et al. reported that the application of Prograf (FK506) combined with mycophenolate mofetil (MMF) and prednisone could help recover the motility and morphology of the sperm in kidney transplantation recipients (27). Similarly, in our study, all of the patients were treated with cyclosporine, mycophenolate mofetil, and prednisone, which had no negative effect on semen parameters. Another study by Haberman et al. showed that in young patients who had cyclosporine A therapy followed by renal transplantation, most semen parameters and testicular hormonal functions were normal and it seems that Cyclosporine A does not adversely affect the fertility in men (28). Lessan-Pezeshki and and Ghazizadeh evaluated the hormonal profile and semen parameters in CKD patients before and after renal transplantation. They indicated that semen volume, sperm count, and motility were improved after transplantation; additionally, prednisone and corticosteroids could not affect semen parameters (29).
In addition, in the present study, we evaluated the damage of sperm DNA in patients with kidney transplantation by toluidine blue and aniline blue. There was a significant improvement in maturity of sperm DNA after kidney transplantation that could probably affect the quality of the embryo. Additionally, there was a significant enhancement in semen parameters such as volume, sperm count, and motility that were similar to other studies (24, 25, 29). None of these studies focused on sperm DNA after kidney transplantation and our study was the first one.
On the other hand, with respect to simple and fast staining methods (TB and AB staining), we offer these techniques for analysis of sperm DNA. These methods could explain infertility in cases with normal parameters of semen in addition to estimate the rate of oocyte fertilization by chromatin structure and condensation of sperm.
5.1. Conclusion
Kidney transplantation could improve semen parameters such as volume, sperm count, motility, chromatin structure and condensation of sperm. Both toluidine blue and aniline blue staining methods are simple and fast techniques that could help to identify maturity of sperm DNA.