1. Background
Cisplatin is an effective and widely used treatment for solid tumors (1, 2) with dose-dependent nephrotoxicity, ototoxicity, and neurotoxicity side effects (3-5). Acute kidney injury (AKI) is an important dose-limiting complication of Cisplatin containing chemotherapy regimens, which is associated with higher mortality and morbidity (6, 7). Nephrotoxicity is often characterized by electrolyte disturbances, increased serum creatinine, and decreased Glomerular filtration rate (GFR) (8). Multiple mechanisms contribute to Cisplatin-associated renal tubular damage, such as oxidative stress, vasoconstriction, inflammation, and apoptosis (9, 10).
Renal function monitoring is crucial in patients, who receive Cisplatin, because about 30% experience AKI, despite applying nephroprotective measures, such as pre-hydration and mannitol administration (8). Serum creatinine (Cr) rises following AKI, yet researchers have been looking for biomarkers that rise in earlier stages of renal impairments (11). Neutrophil gelatinase-associated lipocalin (NGAL) is one of the promising candidate biomarkers (12). Furthermore, NGAL is expressed at low concentration in the lungs, kidneys, liver, spleen, and colon tissues. It’s monomeric 25-kDa form is secreted by damaged renal tubular cells in urine (13). Animal models show NGAL increases in very early stages of kidney injury, proceeding to Cr increase (14). The role of NGAL in predicting AKI has been studied in a wide range of clinical conditions (13, 15-18). Some studies even compare the role of NGAL in predicting AKI to troponin in myocardial Infarction (13).
Given that Cisplatin AKI results from tubular damage, urine NGAL measurement could be a noninvasive, clinically convenient, and reliable approach for timely diagnosis and treatment of renal impairment in patients receiving Cisplatin containing chemotherapy (13, 15, 17-19). Since urinary NGAL can predict renal injury better than plasma NGAL (16), the researchers chose urine as a sample.
2. Objectives
This longitudinal study aimed at comparing urine NGAL with other conventional biomarkers (serum Cr, BUN) and GFR in predicting Cisplatin-induced AKI.
3. Methods
The study consisted of 35 participants with various types of malignancy, who received Cisplatin containing chemotherapy. They were recruited from referrals to Department of Oncology, Baqiyatallah Hospital, Tehran (Iran) between August 2013 and September 2014. To be included in the study, informed volunteers were required to have baseline normal renal function tests, receive no other nephrotoxic treatment but Cisplatin, and present with no comorbidities that affect renal function (e.g. sepsis, urinary tract infection or hypotension).
Blood and urine samples, for baseline measurements, were collected from each participant before chemotherapy. Blood sampling was repeated on days one, three, six, and thirty after receiving Cisplatin to follow up with changes in BUN and serum Cr levels. Two subsequent urine samples were collected 6 and 24 hours after Cisplatin treatment for NGAL measurements.
The renal function was estimated by calculating the GFR based on Cockcroft-Gault equation: GFR (mL/min/1.73 m2) = (140 - age) × (weight kg)/72 × Cr (× 0.85 in females). According to the Kidney disease improving global outcomes (KDIGO) criteria, AKI was defined as either increase in serum creatinine concentration by ≥ 0.3 mg/dL within 48 hours or ≥ 50% than the baseline within seven days (15).
Since participants were to receive treatment for various types of cancer, it was not possible to administer a universal chemotherapy regimen. However, all patients received 50 mg/m2 of Cisplatin and were pre-hydrated with two to three liters of isotonic saline.
Quantitative and qualitative data were summarized as mean ± SD and frequency (%), respectively. The Kolmogorov-Smirnov test was used to assess normality. Mann-Whitney test was used to compare the medians. Chi-square and Fisher’s exact tests were applied for categorical variable comparison. Marginal linear regression model with GEE estimates was used to analyze changes in the variable over time. Receiver operating characteristic (ROC) curves were constructed to test the ability of NGAL, BUN, Cr, and eGFR to predict AKI at different points of time.
Analysis was performed with the IBM SPSS Statistics for Windows (version 20.0 Armonk, NY: IBM Corp) and STATA statistical software (Release 12. College Station, TX: StataCorp LP). P < 0.05 was considered statistically significant.
The study protocol was approved by Institutional Ethic Committee of Baqiyatallah University of Medical Sciences. The participants enrolled upon giving informed written consents.
4. Results
The study consisted of 35 participants (14 males and 21 females) with mean ± SD age of 65.15 ± 14.14. The most common type of malignancy was Esophageal Adenocarcinoma (N = 11, 31%). Six (17.1%) patients developed AKI after receiving Cisplatin treatment. The severity of kidney injury was exclusively limited to Stage 1, with Cr level returning to baseline within a month; except in one patient. There were no significant differences between AKI and non-AKI patients regarding age (P = 0.37), gender (P = 0.15), and type of malignancy (P = 0.99).
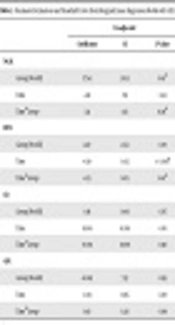
Patients with AKI had significantly higher serum Cr on day three (P = 0.01) and day six (P = 0.03), compared to the non-AKI group. Day 3 BUN level was significantly higher in AKI patients (P = 0.01) as well. Table 1 summarizes and compares demographics and lab findings in AKI and non-AKI patients.
| Total (N = 35) | AKI (N = 6) | Non-AKI (N = 29) | P Valueb | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 65.15 ± 14.14 | 65 (26.75) | 67 (24) | 0.37 |
| BMI, kg/m2 | 25.99 ± 4.92 | 24.65 (6.30) | 27.20 (10.70) | 0.39 |
| Weight, kg | 66.09 ± 9.97 | 67 (12) | 69.50 (16.25) | 0.52 |
| NGAL, ng/mL | ||||
| Baseline | 83.65 ± 112.24 | 22 (17.25) | 29.60 (194.65) | 0.77 |
| Hour 6 | 77.11 ± 93.34 | 28.70 (30.88) | 34.70 (182.78) | 0.72 |
| Hour 24 | 61.19 ± 55.80 | 47.50 (46.95) | 36.20 (130.20) | 0.92 |
| Cr, mg/dL | ||||
| Baseline | 1.01 ± 0.26 | 1.05 (0.45) | 0.95 (0.42) | 0.68 |
| Day 1 | 1.05 ± 0.23 | 1.10 (0.48) | 0.95 (0.25) | 0.11 |
| Day 3 | 1.05 ± 0.21 | 1.25 (0.25) | 1 (0.20) | 0.01 |
| Day 6 | 1.06 ± 0.22 | 1.20 (0.45) | 1 (0.25) | 0.03 |
| Day 30 | 1.05 ± 0.25 | 1.15 (0.63) | 1 (0.40) | 0.40 |
| BUN, mg/dL | ||||
| Baseline | 17.53 ± 7.37 | 15.50 (8.00) | 16.50 (5.50) | 0.86 |
| Day 1 | 21.46 ± 7.21 | 24.50 (11.00) | 19.50 (7.50) | 0.09 |
| Day 3 | 25.46 ± 7.91 | 29.50 (13.50) | 22.50 (11.00) | 0.01 |
| Day 6 | 27 ± 11.39 | 37 (21.50) | 27 (13.50) | 0.22 |
| Day 30 | 17.08 ± 3.16 | 17 (4.50) | 17 (5) | 0.84 |
| eGFR, mL/min/1.73 m2 | ||||
| Baseline | 62.32 ± 19.28 | 60.85 (35.47) | 56.95 (20.55) | 0.30 |
| Day 1 | 59 ± 15.57 | 50.55 (40.58) | 57.65 (16.55) | 0.68 |
| Day 3 | 58.96 ± 15.78 | 52.75 (17.39) | 57.65 (12.18) | 0.52 |
| Day 6 | 57.58 ± 10.71 | 43.60 (27.90) | 57.40 (12.80) | 0.12 |
| Day 30 | 57.02 ± 10.34 | 58.20 (20.22) | 57.40 (14.00) | 0.72 |
aValues are expressed as mean ± SD or median (Inter Quartile Range).
bMann-Whitney test.
According to unadjusted marginal linear regression model, the mean increase in NGAL level per hour was 3.31 (0.05 to 6.57) times higher in AKI patients compared with non-AKI group (0.04). When adjusted for age, gender, and BMI, the mean NGAL increase per hour was 3.32 (0.52 to 7.16) times higher in AKI patients (P = 0.08). Table 2 shows parameter estimations and standard errors from marginal linear regression model.
| Unadjusted | Adjusteda | |||||
|---|---|---|---|---|---|---|
| Coefficient | SE | P Value | Coefficient | SE | P Value | |
| NGAL | ||||||
| Group (No AKI) | -77.41 | 30.15 | 0.01b | -77.88 | 42.74 | 0.08 |
| Time | -2.21 | 1.53 | 0.14 | -2.27 | 1.84 | 0.21 |
| Timeb Group | 3.31 | 1.63 | 0.04b | 3.32 | 1.92 | 0.08 |
| BUN | ||||||
| Group (No AKI) | 4.67 | 2.64 | 0.07 | 1.77 | 3.36 | 0.59 |
| Time | -0.10 | 0.02 | < 0.001b | -0.10 | 0.02 | < 0.001b |
| Timeb Group | -0.13 | 0.05 | 0.01b | -0.12 | 0.06 | 0.04b |
| CR | ||||||
| Group (No AKI) | 0.16 | 0.09 | 0.07 | 0.07 | 0.07 | 0.35 |
| Time | 0.001 | 0.001 | 0.88 | 0.001 | 0.001 | 0.88 |
| Timeb Group | -0.001 | 0.005 | 0.90 | -0.001 | 0.005 | 0.91 |
| GFR | ||||||
| Group (No AKI) | -0.005 | 7.23 | 0.99 | -4.07 | 5.32 | 0.44 |
| Time | -0.01 | 0.06 | 0.88 | -0.002 | 0.06 | 0.97 |
| Timeb Group | 0.01 | 0.20 | 0.94 | 0.04 | 0.19 | 0.81 |
aModel adjusted for sex, age, BMI.
bSignificant at level 0.05.
The area under curve (SD) for urine NGAL measured six hours after Cisplatin treatment (adjusted for age, gender, and BMI) was 0.94 (0.04) (P = 0.06). Table 3 summarizes the results of ROC curve analysis.
| Area Under the Curve (SE) | Significancy | |
|---|---|---|
| NGAL | ||
| Not adjusted | χ2 = 4.75, P = 0.02 | |
| Hour 6 | 0.37 (0.13) | |
| Hour 24 | 0.66 (0.11) | |
| Adjusted | χ2 = 3.51, P = 0.06 | |
| Hour 6 | 0.94 (0.04) | |
| Hour 24 | 0.81 (0.08) | |
| Cr | ||
| Not adjusted | χ2 = 3.20, P = 0.36 | |
| Day 1 | 0.70 (0.10) | |
| Day 3 | 0.81 (0.10) | |
| Day 6 | 0.80 (0.09) | |
| Day 30 | 0.63 (0.16) | |
| Adjusted | χ2 = 8.25, P = 0.04 | |
| Day 1 | 0.86 (0.10) | |
| Day 3 | 0.92 (0.05) | |
| Day 6 | 1.00 (0.00) | |
| Day 30 | 0.82 (0.09) | |
| eGFR | ||
| Not adjusted | χ2 = 3.32, P = 0.34 | |
| Day 1 | 0.44 (0.15) | |
| Day 3 | 0.41 (0.12) | |
| Day 6 | 0.27 (0.17) | |
| Day 30 | 0.44 (0.15) | |
| Adjusted | χ2 = 10.12, P = 0.01 | |
| Day 1 | 0.84 (0.11) | |
| Day 3 | 0.94 (0.04) | |
| Day 6 | 1.00 (0.00) | |
| Day 30 | 0.81 (0.09) | |
| BUN | ||
| Not adjusted | χ2 = 8.65, P = 0.03 | |
| Day 1 | 0.72 (0.12) | |
| Day 3 | 0.81 (0.08) | |
| Day 6 | 0.68 (0.14) | |
| Day 30 | 0.53 (0.14) | |
| Adjusted | χ2 = 2.80, P = 0.42 | |
| Day 1 | 0.92 (0.04) | |
| Day 3 | 0.93 (0.05) | |
| Day 6 | 0.82 (0.08) | |
| Day 30 | 0.80 (0.09) |
5. Discussion
Less than one-fifth of study participants developed AKI according to KDIGO criteria. The frequency of Cisplatin induced AKI varies extensively between studies. Saadat et al. showed in another study that nine out of 80 participants (11%) experienced 25% to 30% reduction in GFR after receiving Cisplatin (20). Shahbazi et al., and Moon et al., estimated that less than 10% of patients develop AKI after treatment with Cisplatin (21, 22). Cisplatin-induced nephrotoxicity has been reported in about 30% of patients in separate studies conducted by Miller et al., dos Santos et al., Karasawa and Steyger, and Lin et al. (3-5, 23).
In the present study neither group of patients, regarding AKI development, showed significant changes in urine NGAL levels after Cisplatin treatment compared to baseline. Sterling et al. reported that the level of urinary NGAL did not significantly increase after drug infusion in children, who received cisplatin containing chemotherapy regimen (24). Kos et al. reported no significant correlation between serum NGAL and serum Cr levels in patients receiving Cisplatin-based chemotherapy (25). On the other hand, Mishra et al., Bennett et al., and Nickolas et al. reported in separate studies that NGAL level increased 24 to 48 hours ahead of serum Cr rise in patients with AKI (26-28). The small size of AKI group is the statistical explanation of observed absence of differences in the present study. From pathophysiological perspective, these findings might be explained by facts that multiple mechanisms contribute to AKI in participants, which do not result in urinary NGAL increases as much as tubular damages. Besides, NGAL increase is directly associated with severity of kidney injury, while AKI in study participants was limited only to stage 1.
In addition to comparing biomarker levels after Cisplatin treatment with corresponding baseline values, the researchers examined changes in biomarker levels overtime. The NGAL increase per hour in AKI patients was considerably higher than the non-AKI group. Though the size of study groups was not enough to show a statistically significant difference after adjustment.
When adjusted for age, gender, and BMI, Cr and eGFR measured on day six were able to classify patients with and without AKI with 100% precision. However, at this point of time, when AKI is already established, the test is not sufficient for predictive and preventive purposes. Despite marginal statistical significance, which could be explained by the small sample size, the findings suggest that Urine NGAL measured 24 hours after Cisplatin treatment could predict AKI accurately and timely enough to prevent serious damages. Shahbazi et al. suggested urinary NGAL/Cr ratio (ng/mg) as a marker of early cisplatin-induced AKI. They even demonstrated that the ratio calculated based on 24 hours post Cisplatin infusion NGAL and Cr, is more accurate than serum creatinine in predicting AKI (22). Lin et al.’s study showed that urinary NGAL is a better predictor of AKI than albuminuria or urinary cystatin C levels (23). Gaspari et al. reported that NGAL increase precedes AKI by 4.5 days and NGAL increase on day two after Cisplatin treatment is an independent predictor of AKI nephrotoxicity (29).
5.1. Conclusion
The proportion of patients, who developed Cisplatin-associated AKI was not large enough to show a statistically significant difference between AKI and non-AKI groups, yet the findings suggest that AKI patients show higher rate of NGAL increase per hour, and urine NGAL measured 24 hours after Cisplatin treatment could be a promising candidate biomarker to predict AKI in patients receiving Cisplatin containing chemotherapy. Studies with larger groups of participants, e.g. multicentral studies or meta-analysis for pooling research findings might help resolve controversies.
5.2. Study Limitations
This study was conducted on a small group of participants with considerable heterogeneity regarding type of cancer and history of chemotherapy. Therefore, the correlation between type of cancer with biomarkers’ level change and AKI development could not be examined. It was not possible to confirm whether the NGAL increase was permanent or transient. However, previous studies suggest the phenomenon as permanent (30, 31).