1. Background
The prevalence of idiopathic nephrotic syndrome (NS) is about 1.15 to 16.9 per of 100000 children that depends on race and geographical location. The root cause of this disease is unknown, but its pathogenesis includes various factors such as immunity, systemic features, and structure abnormalities (1-4). The histopathology of idiopathic NS included minimal change nephrotic syndrome (MCNS), mesangial proliferative glomerulonephritis (MesPGN), and focal segmental glomerulosclerosis (FSGS) (5). In NS, serum IgM level increases while immunoglobulin G (IgG) and immunoglobulin A (IgA) levels decrease. The dysfunction of T-cell can reverse immunoglobulin (IgM) and IgG syntheses (6). Prognosis of long-term kidney outcome is very important since steroid resistance is a future risk of chronic or end-stage kidney disease (7-10). Steroid-resistant nephrotic syndrome (SRNS) in patients with idiopathic NS is diagnosed based on the lack of complete remission, despite treatment with steroids (11). SRNS is related to many factors including gene, histopathologic type of glomerular lesion, and treatment adherence in children with idiopathic NS (12-15). Using a number of clinical and subclinical factors to predict the SRNS in children is a problem that is mentioned in some studies, but not fully (16-18).
2. Objectives
The current study aimed at investigating the role of serum IgG and IgM levels, and IgG/IgM ratio on admission in the prediction of SRNS in pediatric patients with idiopathic NS.
3. Methods
3.1. Patients
A retrospective chart analysis was performed to identify new-onset NS in all children admitted to the Department of Nephrology and Hemodialysis, National Children’s Hospital, Hanoi, Vietnam. The approval of the hospital Ethics Committee and informed consent of all patients’ parents were obtained to conduct the study. In the study, 41 patients were selected based on the following criteria: A fulfilled definition of NS, age range 5 to 16 years (to exclude congenital NS and apply renal biopsy in case of steroid-resistance), untreated idiopathic NS, and minimum eight weeks of follow-up. All patients with non-nephrotic proteinuria, congenital NS, or NS due to other causes such as metabolic diseases, infection, malignant tumors, cardiovascular disease, and already treated ones were excluded.
Patients were asked for medical history, examination and the routine tests such as urine 24-hour protein, serum albumin, and complete blood count were performed. Serum IgA, IgM, and IgG levels were measured in all patients.
3.2. Definitions and Treatment
By definition, NS comprises heavy proteinuria ≥ 40 mg/h/m2, hypoalbuminemia (serum albumin ≤ 2.5 g/dL), hypercholesterolemia, and edema (19). The treatment of NS followed the guideline of KDIGO 2012 (11, 19). For the first four weeks, prednisone therapy 60 mg/m2/day was chosen (not more than 80 mg/day). The patients’ responses to treatment were defined (11, 19) as a decrease or absence of proteinuria in three consecutive days; steroid sensitivity was defined as a remission within eight weeks of treatment; steroid resistance (no response) was defined as no response to the initial eight-week of steroid treatment. In the current study, the patients were diagnosed with the SRNS with urine protein of 40 ≥ mg/h/m2 or morning urine ≥ 100 mg/dL; or continuation of dip stick for three days in spite of taking enough steroid doses in specific time according to the guidelines on the applied treatment regimen (19).
3.3. Determination of the Serum Level of IgA, IgG, and IgM
Blood samples were taken from patients immediately after admission to determine the level of serum IgA, IgG, and IgM (before any treatment). Serum IgG and IgM levels were measured based on the enzyme-linked immunosorbent assay (ELISA) technique with the kit supplied by Genway (Biotech, Inc., GenWay Biotech, USA). Serum IgA was measured using ELISA kit of Immunodiagnostic Systems (GmbH, Germany). Normal values of serum IgA, IgG, and IgM in Vietnamese children is not known; therefore, 28 healthy age- and gender-matched children were selected to estimate normal values of serum IgA, IgG, and IgM.
3.4. Kidney Biopsy and Determination of Pathological Lesion
Since kidney biopsy was not common for Vietnamese children, it was performed only in the subjects with steroid resistance. Minimal change nephrotic syndrome (MCNS) was defined as the expansion of mesangium, without kidney injury under the light microscopy. Mesangial proliferative glomerulonephritis (MesPGN) was described as the expansion of mesangial matrix and the proliferation of mesangial cells. Focal segmental glomerulosclerosis (FSGS) was described as the presence of at least one glomerulus with segmental sclerotic lesions. Membranoproliferative glomerulonephritis (MPGN) was defined as the proliferation of mesangial cells together with immune deposits on basement membrane. Finally, membranous glomerulonephritis (MGN) was characterized by the presence of basement membrane immune deposits.
3.5. Statistical Analysis
All data were analyzed by Statistical Package for Social Science (SPSS) version 20.0 (IBM, USA). Continuous variables were described as mean and standard deviation. Two or more continuous variables were analyzed by t-test or one-way ANOVA. Categorical variables were described as frequency and percentage. Two or more categorical variables were analyzed by chi-square test. Receiver operating characteristic (ROC) curve was drawn in order to predict steroid-resistant NS in patients with idiopathic NS.
4. Results
According to baseline characteristics, the median age of children with idiopathic NS was eight years; 78% of them were male. Up to 43.9% of the patients had oliguria, while the median value of urine 24-hour protein was 8.23 g. The prevalence of SRNS in the current study was 46.3% (Table 1).
| Clinical Characteristics and Laboratory Parameters | Mean ± SD/Median (Range) | No. (%) |
|---|---|---|
| Age, y (min - max) | 8 (6 - 10) (5-15) | N/A |
| Number of male | N/A | 32 (78) |
| Hypertension | N/A | 8 (19.5) |
| Anemia | N/A | 10 (24.4) |
| Blood urea (mM/L) | 4.69 (3.75 - 7.01) | N/A |
| GFR (mL/min) | 89.59 ± 21.41 | N/A |
| Oliguria | N/A | 18 (43.9) |
| Serum protein (g/L) | 49.43 ± 12.33 | N/A |
| Serum albumin (g/L) | 19.7 (16.24 - 34.42) | N/A |
| Urine protein (g/24h) | 8.23 (5.31 - 17.84) | N/A |
| Serum cholesterol (mM/L) | 10.08 (5.6 - 13.48) | N/A |
| Serum triglyceride (mM/L) | 3.74 ± 1.23 | N/A |
| Steroid-resistant NS | N/A | 19 (46.3) |
Abbreviations: GFR, glomerular filtration rate; N/A, not available; NS, nephrotic syndrome.
The current study included 41 children with NS and 28 healthy children as controls. The median value of serum IgA in apparently healthy individuals was 2.23 g/L. The median value of IgG level was 10.6 g/L, which was significantly higher than that of the NS group, P < 0.001. However, there was no significant difference in the serum IgM level between the two groups. The ratio of serum IgG/IgM in the control group was significantly higher than that of the NS group; P < 0.001 (Table 2).
| NS (N = 41) | Control (N = 28) | P Value | |
|---|---|---|---|
| Serum IgA (g/L) | 1.15 (0.99 - 1.64) | 2.23 (2.02 - 2.74) | < 0.001 |
| Serum IgG (g/L) | 2.23 (1.11 - 5.35) | 10.6 (9.81 - 12.59) | < 0.001 |
| Serum IgM (g/L) | 1.7 (1.3 - 2.06) | 1.29 (1.16 - 1.95) | 0.071 |
| Serum IgG/IgM ratio | 1.48 (0.56 - 3.69) | 8.06 (6.15 - 9.6) | < 0.001 |
Abbreviation: NS, nephrotic syndrome.
aValues are expressed as median (range).
According to the baseline characteristics of patients with and without steroid-resistance (Table 3), no significant differences were observed between the study groups in terms of the mean age, male-to-female ratio, incidence of hypertension, anemia, GFR, oliguria, and serum IgM level in the subjects with SRNS or SSNS (P > 0.05). The median value of urine 24-hour protein was higher in children with SRNS than the SSNS group (P = 0.009). The median level of serum IgA and IgG, and IgG/IgM ratio were significantly lower in the SRNS group than the SSNS (P = 0.04, < 0.001, and < 0.001, respectively).
| Clinical Characteristics and Laboratory Parameters | SSNS (N = 22) | SRNS (N = 19) | P Value |
|---|---|---|---|
| Age, y | 7.5 (6 - 10) | 8 (6 - 11) | 0.833c |
| Number of male | 18 (81.8) | 14 (73.7) | 0.709d |
| Hypertension | 4 (18.2) | 4 (21.1) | 0.817d |
| Anemia | 5 (22.7) | 5 (26.3) | 0.79d |
| Blood urea (mM/L) | 4.75 (3.87 - 6.75) | 4.69 (3.66 - 7.17) | 0.774c |
| GFR (mL/min) | 89.77 ± 18.05 | 89.37 ± 25.27 | 0.953b |
| Oliguria | 11 (50) | 7 (36.8) | 0.397d |
| Serum protein (g/L) | 54.89 ± 12.85 | 43.11 ± 8.16 | 0.001b |
| Serum albumin (g/L) | 27.24 (16.95 - 40.69) | 17.1 (14.66 - 20.12) | 0.009c |
| Urine protein (g/24h) | 5.99 (5.07 - 11.87) | 15.03 (7.17 - 23.78) | 0.007c |
| Cholesterol (mM/L) | 6.84 (4.92 - 11.15) | 12.83 (9.89 - 14.41) | 0.006c |
| Triglyceride (mM/L) | 3.3 ± 1.06 | 4.26 ± 1.24 | 0.011b |
| Serum IgA (g/L) | 1.31 (1.1 - 1.8) | 1.11 (0.87 - 1.35) | 0.04c |
| Serum IgG (g/L) | 4.39 (2.96 - 9.34) | 1.03 (0.9 - 1.67) | < 0.001c |
| Serum IgM (g/L) | 1.61 (1.24 - 1.95) | 1.84 (1.36 - 2.27) | 0.102c |
| Serum IgG/IgM ratio | 2.72 (1.83 - 6) | 0.57 (0.46 - 1.07) | < 0.001c |
Abbreviations: GFR, glomerular filtration rate; NS, nephrotic syndrome; SRNS, steroid-resistant nephrotic syndrome; SSNS: steroid-sensitive nephrotic syndrome.
aValues are expressed as mean ± SD, median (range), or No. (%).
bt-test.
cThe Mann-Whitney U test.
dChi-square.
In the current study, MesPGN had the highest prevalence, while MGN had the lowest prevalence. The prevalence of MCNS in the subjects with SRNS was 15.8% (Table 4).
| No. (%) | |
|---|---|
| MCNS | 3 (15.8) |
| FSGS | 5 (26.3) |
| MesPGN | 8 (42.1) |
| MPGN | 2 (10.5) |
| MGN | 1 (5.3) |
| Total | 19 (100.0) |
Abbreviations: FSGS, focal segmental glomerulosclerosis; MCNS, minimal change nephrotic syndrome; MesPGN, mesangial proliferative glomerulonephritis; MGN, membranous glomerulonephritis; MPGN, membrnoproliferative glomerulonephritis.
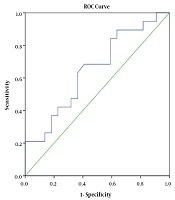
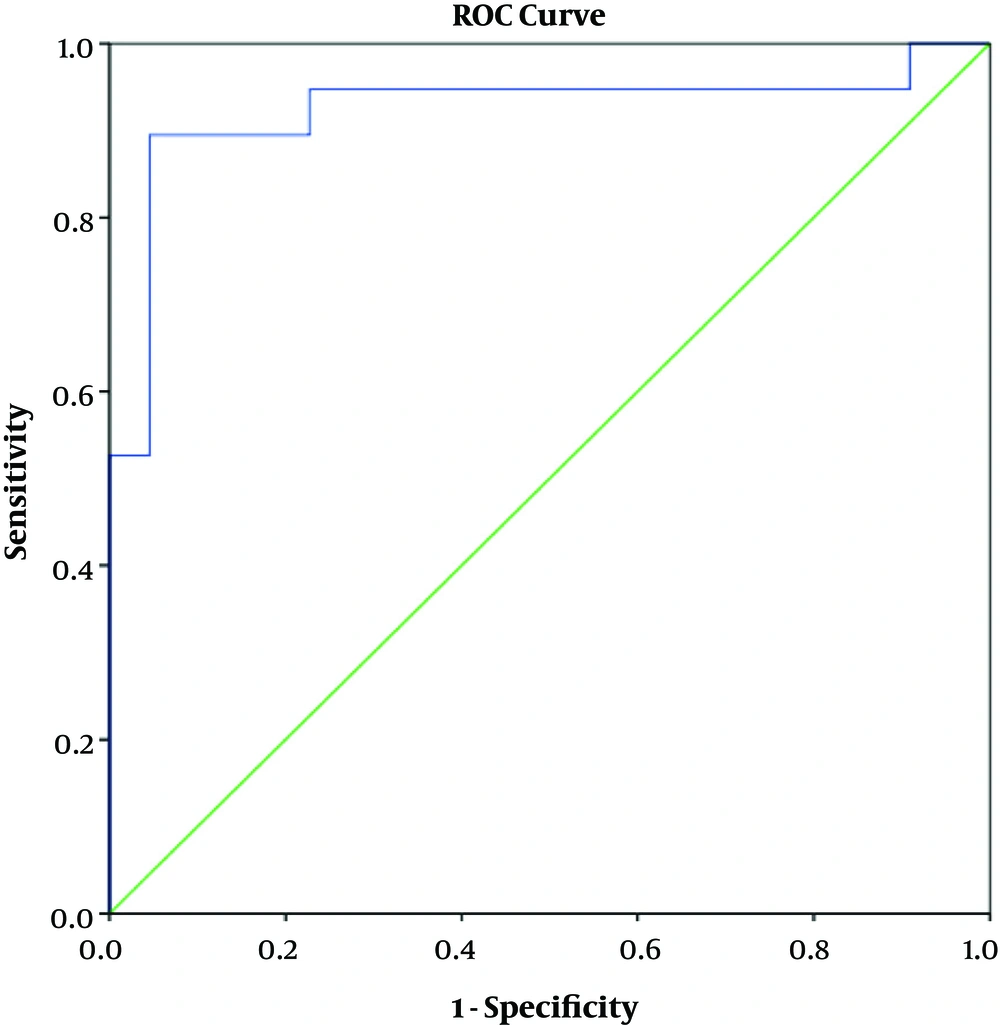
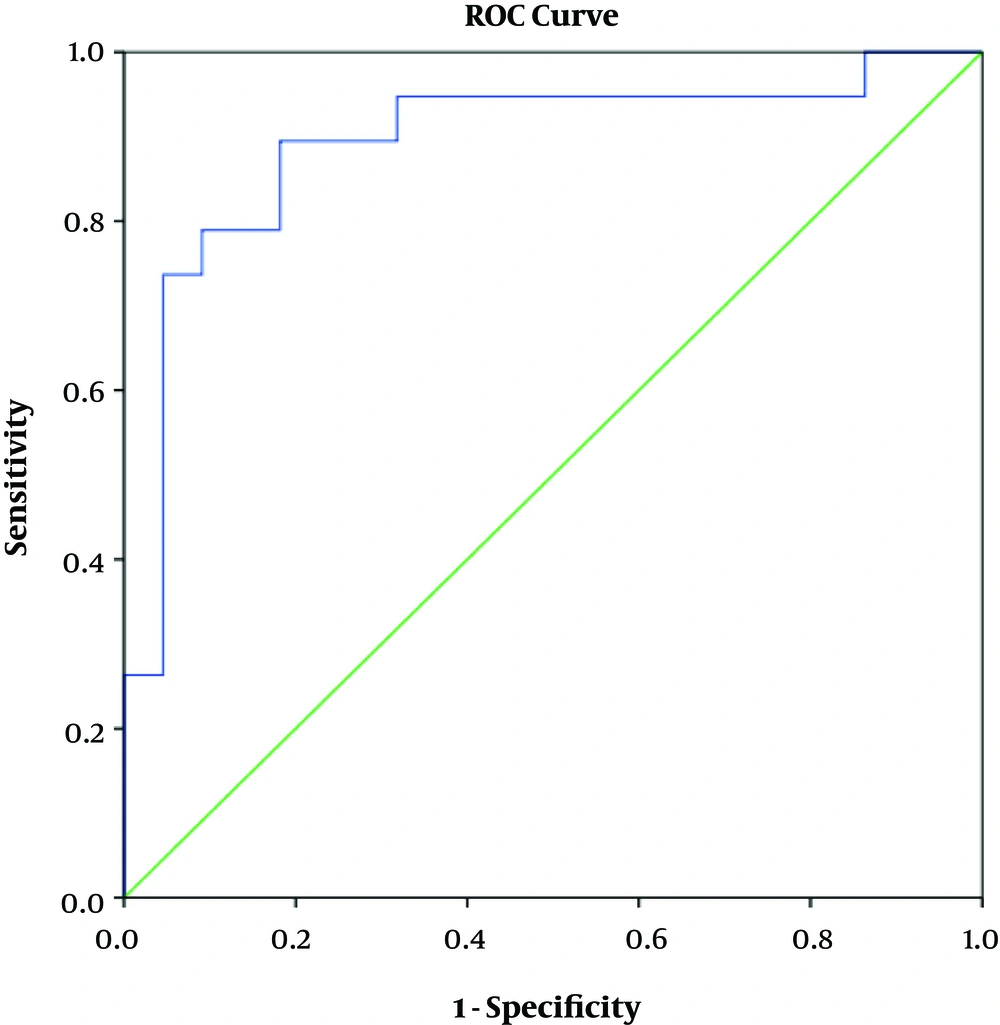
In terms of determining the predictive value of serum IgG, IgM, and IgG/IgM ratio using ROC curve model, the results showed that IgG had a positive predictive value for SRNS (AUC = 0.923, P < 0.001). With the cutoff point of 2.04 g/L, this test had the sensitivity and specificity of 89.5% and 95.5%, respectively (Figure 1). The IgG/IgM ratio also had a predictive value for SRNS (AUC = 0.892, P < 0.001). With the cutoff point of 1.64 g/L, this test had the sensitivity and specificity of 89.5% and 81.8%, respectively (Figure 2). IgM had no predictive value for SRNS (Figure 3).
When baseline demographic characteristics and laboratory findings were compared in patients based on the cutoff point of serum IgG (Table 5) and IgG/IgM ratio (Table 6), it was observed that clinical signs and laboratory findings were worse in patients with serum IgG level and IgG/IgM ratio below the cutoff point, than the ones with the same values above the cutoff point (P < 0.05).
| Clinical Characteristics and Laboratory Parameters | Lower Group (IgG < 2.04 g/L), (N = 14) | Higher Group (IgG ≥ 2.04 g/L), (N = 27) | P Value |
|---|---|---|---|
| Age, y | 7 (5 - 9) | 9 (6 - 10) | 0.143c |
| Number of male | 11 (78.6) | 21 (77.8) | 0.954d |
| Hypertension | 2 (14.3) | 6 (22.2) | 0.543d |
| Anemia | 4 (28.6) | 6 (22.2) | 0.653d |
| Blood urea (mM/L) | 5.3 (4.06 - 7.64) | 4.67 (3.52 - 6.9) | 0.438c |
| GFR (mL/min) | 86.86 ± 24.58 | 91 ± 19.93 | 0.564b |
| Oliguria | 7 (50) | 11 (40.7) | 0.571d |
| Serum protein (g/L) | 41.45 ± 5.83 | 53.57 ± 12.84 | < 0.001b |
| Serum albumin (g/L) | 16.35 (14.02 - 19.5) | 26.24 (17.1 - 40.62) | 0.001c |
| Urine protein (g/24h) | 16.41 (11.6 - 36.62) | 6.34 (5.18 - 12.4) | 0.001c |
| Serum cholesterol (mM/L) | 12.9 (10.61 - 14.7) | 7.25 (4.94 - 11.21) | 0.004c |
| Serum triglyceride (mM/L) | 4.15 ± 1.01 | 3.54 ± 1.3 | 0.137b |
| Serum IgA (g/L) | 1.11 (0.9 - 1.2) | 1.31 (1.09 - 1.79) | 0.071 |
| Serum IgM (g/L) | 1.86 (1.65 - 2.57) | 1.6 (1.21 - 1.97) | 0.026c |
| IgG/IgM ratio | 0.51 (0.44 - 0.57) | 2.52 (1.48 - 5.79) | < 0.001c |
Abbreviations: GFR, glomerular filtration rate; NS, nephrotic syndrome.
aValues are expressed as mean ± SD, median (range), or No. (%).
bt-test.
cThe Mann-Whitney U test.
dChi-square.
| Clinical Characteristics and Laboratory Parameters | Lower Group (IgG/IgM Ratio < 1.64), (N = 21) | Higher Group (IgG/IgM Ratio ≥ 1.64), (N = 20) | P Value |
|---|---|---|---|
| Age, y | 8 (5.5 - 10.5) | 8 (6 - 10) | 0.833c |
| Number of male | 16 (76.2) | 16 (80) | 0.768d |
| Hypertension | 3 (14.3) | 5 (25) | 0.387d |
| Anemia | 6 (28.6) | 4 (20) | 0.523d |
| Blood urea (mM/L) | 4.62 (3.75 - 6.84) | 4.86 (3.63 - 7.07) | 0.764c |
| GFR (mL/min) | 91.76 ± 21.75 | 87.3 ± 21.37 | 0.512a |
| Oliguria | 8 (38.1) | 10 (50) | 0.443d |
| Serum protein (g/L) | 41.03 ± 5.5 | 58.25 ± 11.33 | < 0.001b |
| Serum albumin (g/L) | 16.6 (14.3 - 18.78) | 34.42 (24.47 - 40.87) | < 0.001c |
| Urine protein (g/24h) | 15.03 (8.08 - 23.54) | 5.64 (4.67 - 8.14) | 0.001c |
| Serum cholesterol (mM/L) | 12.8 (10.47 - 14.32) | 5.76 (4.42 - 9.93) | < 0.001c |
| Serum triglyceride (mM/L) | 4.4 ± 1.08 | 3.06 ± 1.01 | < 0.001b |
| Serum IgA (g/L) | 1.11 (0.89 - 1.33) | 1.36 (1.1 - 1.77) | 0.098 |
| Serum IgG (g/L) | 1.19 (0.91 - 1.7) | 5.35 (3.98 - 9.46) | < 0.001c |
| Serum IgM (g/L) | 1.97 (1.71 - 2.59) | 1.33 (1.06 - 1.68) | < 0.001c |
Abbreviations: GFR, glomerular filtration rate; NS, nephrotic syndrome.
aValues are expressed as mean ± SD, median (range), or No. (%).
bt-test.
cThe Mann-Whitney U test.
dChi-square.
5. Discussion
To the best of authors’ knowledge, it was the first study in Vietnam that employed clinical and subclinical indicators to predict steroid resistance both in adult and children patients with primary NS. According to the International Kidney Association guidelines as well as the Vietnamese Ministry of Health, patients diagnosed with primary NS should start steroid therapy. Clinicians should then assess the response of patient to treatment. If the patient showed resistance to steroids, the kidney biopsy should be performed in order to detect the histopathological lesions. In fact in Vietnam, kidney biopsy is not performed in young children. That was why the current study was conducted on patients with the minimum age of five. All of the patients participating in the current study were diagnosed with primary NS for the first time, never screened or treated before. Therefore, the clinical and subclinical manifestations of these patients were very clear and typical of idiopathic NS, especially the high amount of proteinuria (5.31 - 17.84 g/24 hours) and the low amount of serum albumin (Table 1). After eight weeks of treatment and evaluation, it was observed that up to 46.3% of patients had steroid resistance. This high prevalence may be due to two main reasons: first, the National Hospital of Pediatrics was the last line of treatment in the North of Vietnam and the disease was severe in most of the patients. Second, the sample size was not large enough; hence, it might affect the prevalence. It was one of the study limitations; to obtain more conclusive results it is necessary to make an assessment on a larger sample size.
5.1. Characteristics of Serum IgA, IgG, and IgM Levels in Children with NS
The results of the current study showed that the serum IgA and IgG levels in the group of patients with idiopathic NS were lower than those of the control group (P < 0.001). In contrast, serum IgM level was higher in patients than that of the controls, but the difference was not significant. The current study results confirmed the variation of serum IgA, IgG, and IgM levels in patients with idiopathic NS. In 2011, Youssef et al. (20), studied the serum level of IgA, IgM, and IgG in 27 patients with active NS (divided into two subgroups: the mean age of patients was 12.3 years in the steroid-resistant group and 11.6 years in the steroid-sensitive group) compared to 20 healthy children (mean age 12.1 years), the obtained results showed that serum IgA and IgG levels (both subgroups) of patients in the NS group were lower than those of the control group (P < 0.05). This study also showed no significant difference in serum IgM levels between patients with idiopathic NS and the healthy group. There are also many published studies on serum IgA, IgG, and IgM levels in patients with primary NS with similar results to those of the current study (6). The reason may be that in pediatric patients with NS, IgA and IgG with small molecular size are excreted in the urine. However, the serum IgM with a large molecular size cannot pass through the injured slit diaphragm of glomerular basement membranes.
5.2. Steroid Resistance in Vietnamese Children with NS
In the current study, after eight weeks of follow-up in 41 children first diagnosed with idiopathic NS, steroid resistance was observed in up to 46.3% of patients. When comparing clinical and subclinical criteria, it was observed that the group of patients with steroid resistance had lower blood albumin levels, more severe proteinuria, and lipid disorder than the ones with steroid sensitivity. In particular, it was observed that the serum level of IgA, IgG, and IgG/IgM ratio in the group of patients with steroid resistance were significantly lower than those of the ones with steroid sensitivity (P < 0.05). The current study results also coincided with those of previously published authors’ study (20-24). Biopsy was performed on 19 patients with steroid-resistant NS, and histopathological results showed that the rate of FSGS, MesPGN, and MPGN accounted for 78.9% of them. Only 15.8% of the patients had minimal change in NS and 5.3% of them had membranous glomerulonephritis. Steroid resistance in the children with idiopathic NS is related to many factors including the histopathological lesions. Previous studies also confirmed that primary NS in children with steroid resistance was more common in patients with FSGS, MesPGN, and MPGN (14, 25-27). These results showed a significant rate of minimal changes in nephrotic syndrome in the steroid-resistant group (28, 29). The abovementioned studies also showed a general result that mutation in some genes were more common in patients with family-related NS. There are currently no guidelines for the use of molecular biological techniques to diagnose SRNS in clinical practice. However, Varner et al. (29), pointed out a lot of benefits for using molecular biology such as easier diagnosis and prognosis of patients, easier selection of suitable donors, predicting post-transplant outcomes, etc.
5.3. Prediction of Steroid-Resistant NS in Vietnamese Children
The current study results showed that serum IgM levels were not valid to predict steroid resistance in children with primary NS, while serum IgG level and IgG/IgM ratio had this potential (Figures 1 - 3). This result looks reasonable since serum IgM levels do not change in patients with NS, but serum IgG levels are significantly lower in children with NS than in the healthy children. Roy et al. (21), conducted a study on 43 patients with NS (24 with steroid sensitivity and 19 with steroid resistance) and 20 healthy subjects. Serum IgG and IgM levels and IgG/IgM ratio were determined in all patients. The results of their study confirmed the decrease of IgG level and IgG/IgM ratio as a predictive marker for bad prognosis in children with NS. Youssef et al. (20), also confirmed that serum IgG level and IgG/IgM ratio were lower in the children with NS and poor steroid response compared to those of the ones with steroid sensitivity. Serum IG level may decrease due to either T-cell dysfunction or increased urinary excretion of albumin, increased IgG catabolism, and decreased IgG synthesis. Furthermore, since IgG has a lower molecular weight than IgM, it is more likely to be excreted in urine than IgM.
Therefore, IgG/IgM ratio is used to evaluate the most comprehensive change of immunoglobulin in patients with SRNS.
To clarify the value of serum IgG level and IgG/IgM ratio to predict steroid-resistant idiopathic NS in children, the current study compared the group of patients with IgG levels and IgG/IgM ratio below the cutoff point and the group of patients from cutoff point and above (Tables 5 and 6). The obtained results showed a brighter difference between the two subgroups. This suggested possible employment of serum IgG levels and IgG/IgM ratio to predict steroid resistance in children with primary NS.
5.4. Limitations
The limitation of the current study was that it mixed all kinds of histopathology (MCNS, FSGS, MesPGN, MPGN, and MGN) to evaluate patients with SRNS. Furthermore, in the current study, there was no patient below five years old, which affected the steroid responsiveness and immunoglobulin concentration. More studies with larger sample sizes should be conducted to clarify the issue.
5.5. Conclusions
In conclusion, serum IgM showed no significant differences between patients with idiopathic NS compared to healthy subjects. Serum IgA and IgG levels were lower in the NS group than in the control group. Serum IgG level and IgG/IgM ratio could be used as predictive markers to predict a poor response to steroids in pediatric patients first diagnosed with idiopathic NS.