1. Background
Primary membranous nephropathy (PMN) is the most common cause of nephrotic syndrome (NS) in adults. It more frequently affects the middle-aged population (40 - 50), characterized by proteinuria, edema, hypoalbuminemia, and hyperlipidemia, with most patients having circulating autoantibodies (1, 2). The histopathological features of PMN consist of sub-epithelial immune complex deposition and subsequent thickening of the glomerular basement membrane. In fact, the diagnosis of PMN could merely be confirmed by ruling out secondary causes such as autoimmunity (e.g., lupus erythematosus), infections (e.g., HBV, HCV, H. pylori), drugs (e.g., gold, penicillamine), and malignancies (e.g., renal and prostate malignancies) that may lead to excessive antibody production (3, 4). Although the pathogenesis of PMN has not yet been fully understood, recent studies have introduced some target autoantigens on podocytes, which their relevant autoantibodies have been detected in the blood circulation of most PMN patients (5). The most known autoantigen is phospholipase A2 receptor (PLA2R), a member of the mannose receptor family. The anti-PLA2R antibody is undetectable in healthy individuals and has an approximate specificity of 70 to 80% for the diagnosis of PMN. Anti-PLA2R evaluation is routinely performed for both diagnosis and follow-up purposes in clinics, as its presence is generally accompanied by worse prognosis (6). Another autoantigen targeted by autoantibodies in PMN is thrombospondin type 1 domain–containing 7A (THSD7A), which is a membrane-associated N-glycoprotein that mediates endothelial cell migration and tube formation found in almost 5% of PMN cases (2). Lecithin-cholesterol acetyltransferase (LCAT), an enzyme involved in maintaining cholesterol homeostasis, has recently been suggested as another probable target (7). In contrast to the secondary MN, which is accompanied by increased levels of IgG1, IgG2, and IgG3, the prominent autoantibody isotype found in PMN directed against the mentioned antigens is IgG4 (4).
In addition to supportive care including blood pressure control, statin administration for hyperlipidemia, low-protein diet, the use of angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers to minimize proteinuria, salt restriction, and diuretics to control edema, it is suggested that patients with progressive loss of glomerular filtration rate (GFR) or refractory proteinuria undergo immunosuppressive therapy by drugs such as steroids, cyclophosphamide, calcineurin inhibitors (cyclosporine and tacrolimus), and finally rituximab (8). Rituximab has shown to ameliorate steroid-resistant refractory PMN, demonstrated by the reduction of urine protein following its administration (9). Rituximab a chimeric monoclonal anti-CD20 antibody has been supposed to eliminate autoantibody-producing B cells via direct signaling, complement-mediated cytotoxicity (CMC), and antibody-dependent cellular cytotoxicity (ADCC) (10). Another mechanism of rituximab action in treating NS is binding to sphingomyelin-phosphodiesterase acid-like-3b (SMPDL-3b) in podocytes, thus preventing their disruption and subsequent proteinuria (11, 12). Rituximab has also shown to induce considerable T cell depletion, mostly CD4+ cells in Rheumatoid Arthritis (RA) patients (13). In addition, rituximab administration reduces retinoic acid-related orphan receptor γt (RORγt) and interleukin‐22 expression, as well as the number of T helper 17 cells, in synovial tissue of RA patients (14).
Despite various mechanisms suggested for rituximab role in treating PMN, the main route seems to be its direct anti-CD20 effect, which targets B lymphocytes and inhibits autoantibody production by reducing their number and function.
2. Objectives
According to the fact that a wide range of B lymphocytes may carry this marker (15), we aimed to identify which subset is more (or less) frequent in PMN patients and which one is more affected by rituximab administration.
3. Methods
Three groups were enrolled in the present study. They included a healthy control group and two patient groups having the clinical, laboratory, and pathological diagnostic criteria of PMN, comprising either patients on standard treatment (the immunosuppressive drugs have been listed at Table 1) or patients on standard treatment plus rituximab (16). The latter group was studied just before receiving rituximab (pre-rituximab) and two months later (post-rituximab). Therefore, four categories were defined for investigation. Patients’ demographic and laboratory data are summarized in Table 1.
| Group | Standard Treatment | Pre-Rituximab | Post-Rituximab | Healthy Control | P Value |
|---|---|---|---|---|---|
| N | 15 | 10 | 10 | 10 | |
| Age, ya | 48 ± 3.26 | 52.2 ± 3.49 | 52.2 ± 3.49 | 45.8 ± 3.5 | 0.56 |
| Gender ratio (M: F) | 11:4 | 8:2 | 8:2 | 7:3 | |
| Creatininea | 1.25 ± 0.03 | 1.81 ± 0.1 | 1.44 ± 0.1 | 1 ± 0.03 | < 0.0001 |
| BUNa | 36.9 ± 1.74 | 50.4 ± 2.26 | 36.8 ± 1.36 | 19.2 ± 0.93 | < 0.0001 |
| WBCa | 6620 ± 261 | 6890 ± 261 | 4342 ± 341 | 5020 ± 270 | < 0.0001 |
| Total lymphocyte percentagea | 48 ± 2 | 54.1 ± 1.98 | 33.5 ± 1.5 | 42.3 ± 1.47 | < 0.0001 |
| Urine protein, mg/dL | 500 - 800: 12 | 3000 - 4000: 5 | < 300: 2 | ||
| 1000 - 1500: 1 | 4000 - 5000: 3 | < 3000: 8 | |||
| 1500 - 3000: 1 | > 5000: 2 | ||||
| > 3000: 1 | |||||
| Anti-PLA2R | Negative: 15 | Positive: 3 | Positive: 1 | ||
| Negative: 7 | Negative: 9 | - | |||
| IS protocol | |||||
| Pred, Cyclosporin | 12 | 7 | 7 | - | |
| Pred, Cyclosporin, Mycophenolate | 1 | 0 | 0 | ||
| Pred, Tacrolimus | 2 | 2 | 2 | ||
| Pred, Mycophenolate, Tacrolimus | 0 | 1 | 1 |
Abbreviations: IS, immunosuppressor; PLA2R, phospholipase A2 receptor; Post-rituximab, PMN patients on standard treatment + rituximab two months after administration; Pre-rituximab, PMN patients on standard treatment + rituximab before receiving the drug; Pred, prednisone; Standard-treatment, PMN patients on standard treatment (steroid + immunosuppressor)
aValues are expressed as mean ± SEM.
Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-hypaque (inno-train, Germany) gradient. Then, cells were frozen in FBS with 10% DMSO and stored in a liquid nitrogen container until finishing sample collection. Afterward, cells were adjusted to a concentration of 1 × 106 cells/mL and stained with mouse antihuman CD19-Cy5-conjugated antibody (Cytognos, Spain), mouse antihuman CD24-PE-conjugated antibody (Cytognos, Spain), and mouse antihuman CD38-FITC-conjugated antibody (Cytognos, Spain) following the manufacturer’s instruction. Flowcytometry was performed by FACSCalibur (BD FacsCalibur Becton Dickinson, USA). The data were analyzed using Flowjo (FlowJo, USA) and Cell Quest Pro software. Unstained cells from each sample were used as controls.
The data were presented as means ± SEM (standard error of the mean). The comparison between groups was performed by non-parametric Kruskal-Wallis test (SPSS 18.0; SPSS Inc., Chicago, USA). P values of less than 0.05 were considered statistically significant.
4. Results
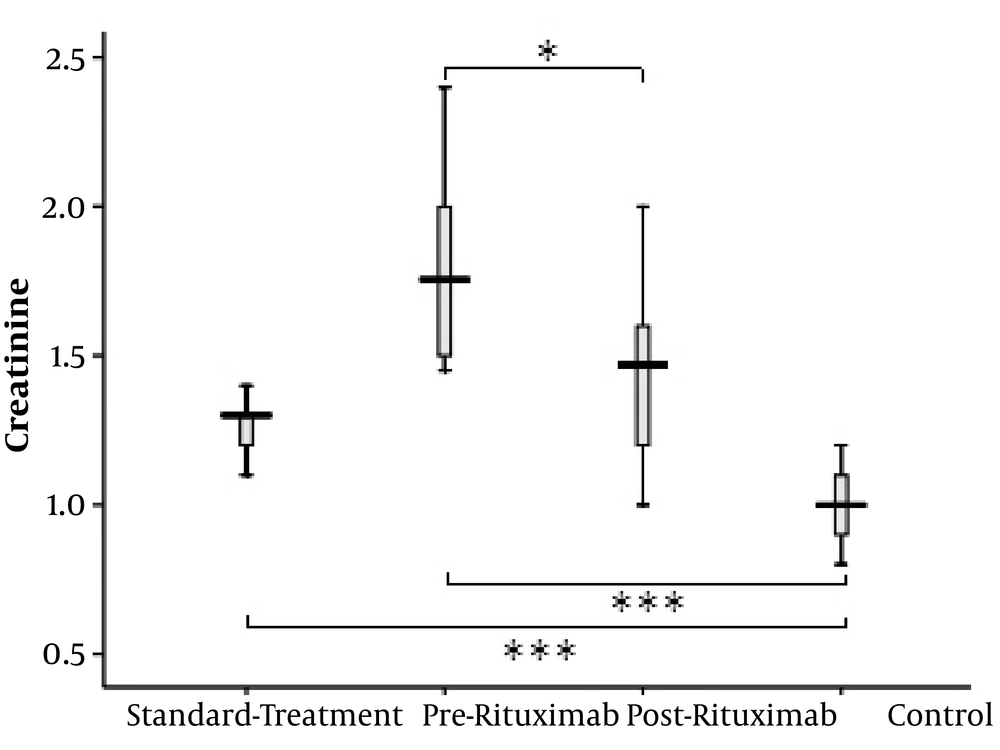
Both standard treatment and rituximab groups had considerably higher creatinine (Cr) levels than healthy controls (P value < 0.0001 for both); however, the patients’ Cr level significantly decreased after receiving rituximab (P value = 0.023) (Figure 1).
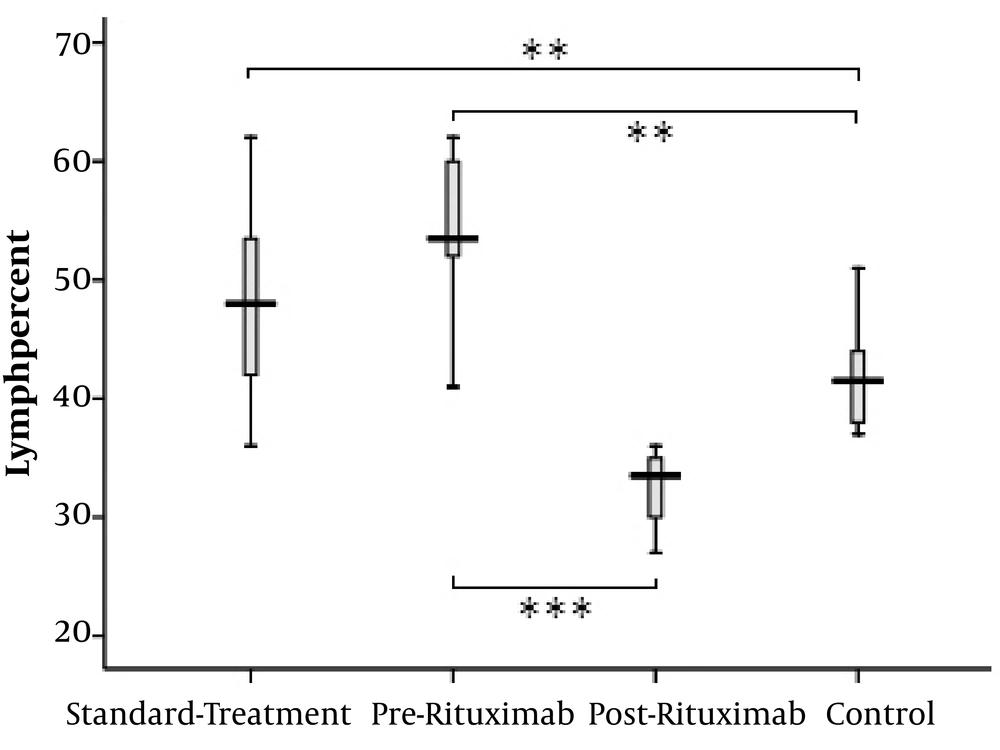
The total lymphocyte percentage was higher in PMN patients receiving either standard treatment or rituximab than in healthy controls (standard-treatment vs. control: P value = 0.001, pre-rituximab vs. control: P value = 0.001). The lymphocyte count significantly decreased following rituximab administration (pre-rituximab vs. post-rituximab: P value < 0.0001) (Figure 2).
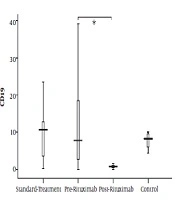
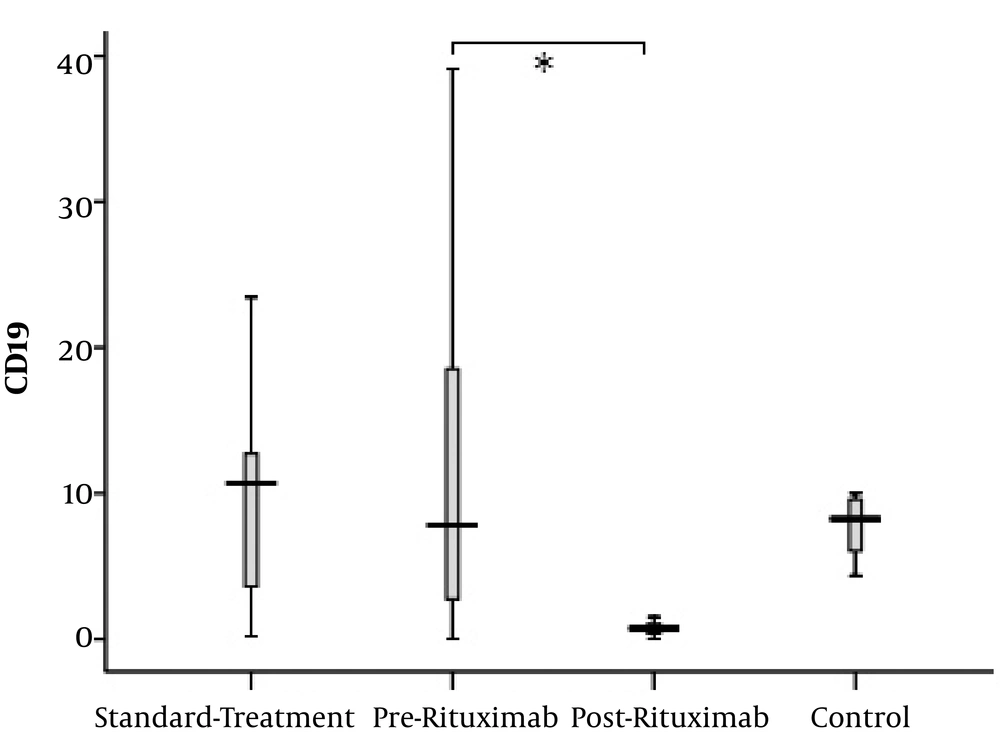
Although there were some patients possessing markedly elevated counts of CD19+ lymphocytes, the CD19+ cell proportion was not significantly different between the control group and both patient groups. However, in post-rituximab analysis, CD19+ cell count showed notable reduction (P value = 0.003) (Figure 3).
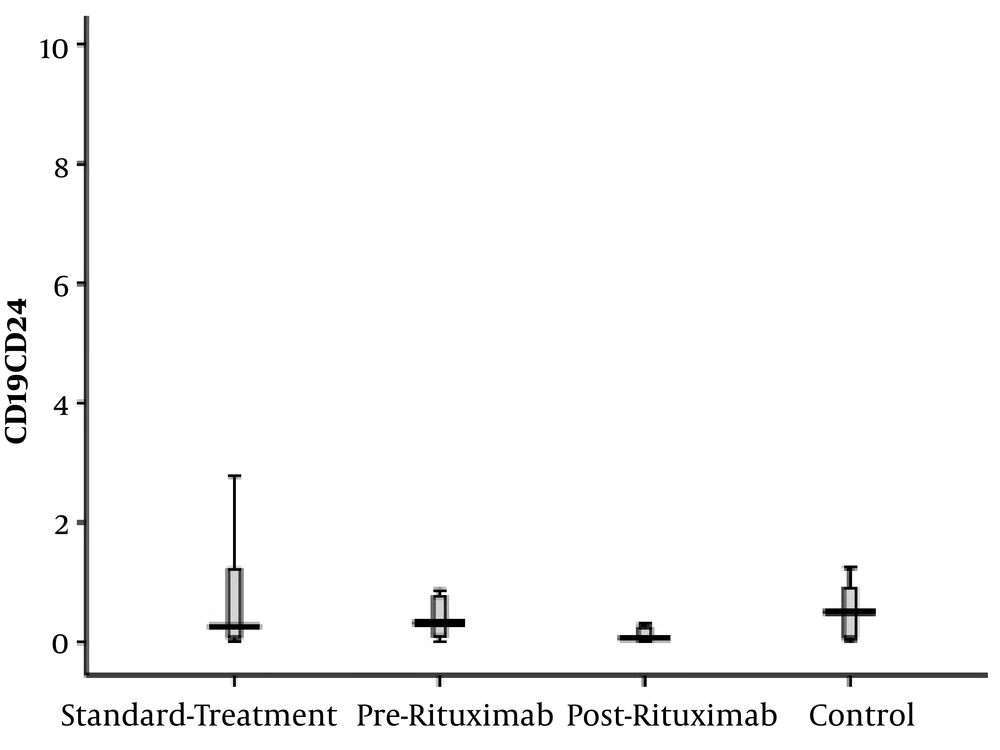
B CD19+CD24+CD38- cells, representing the memory B cell population, did not show any significant difference between healthy controls and patients. Furthermore, the count of these cells did not decrease significantly two months after rituximab administration (Figure 4).
CD19+CD24+CD38- cells frequency (percentage in CD19+ cells) in four groups of standard treatment (steroid + immunosuppressive), pre-rituximab (steroid + immunosuppressive + rituximab), post-rituximab (two months after RTX administration), and healthy controls; *P < 0.05 **P < 0.001 ***P < 0.0001
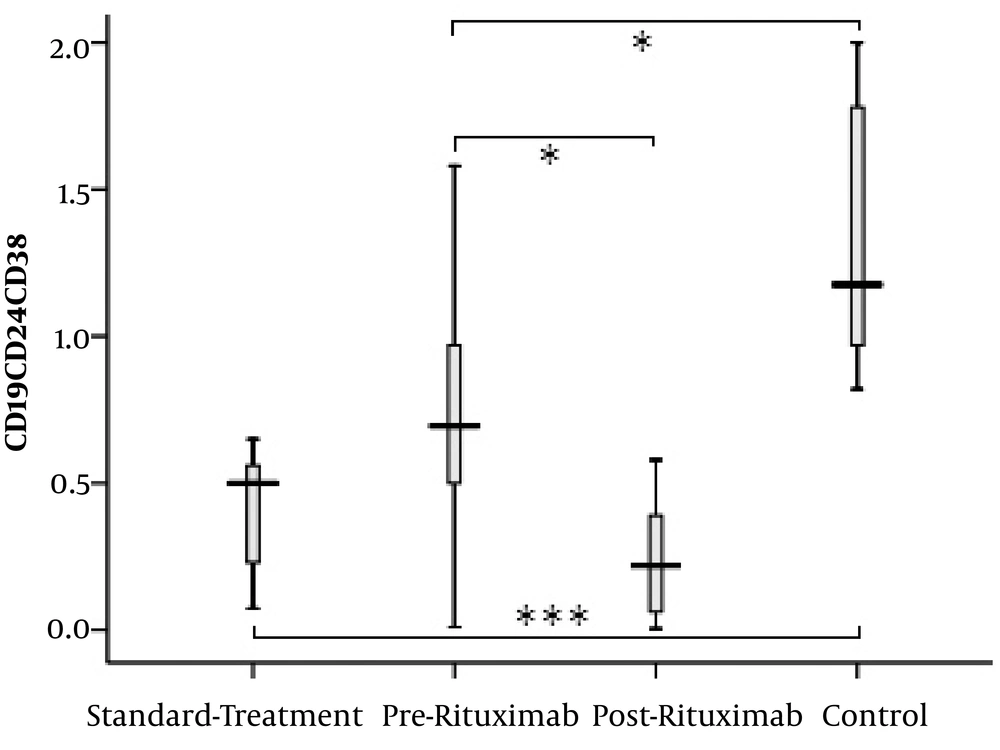
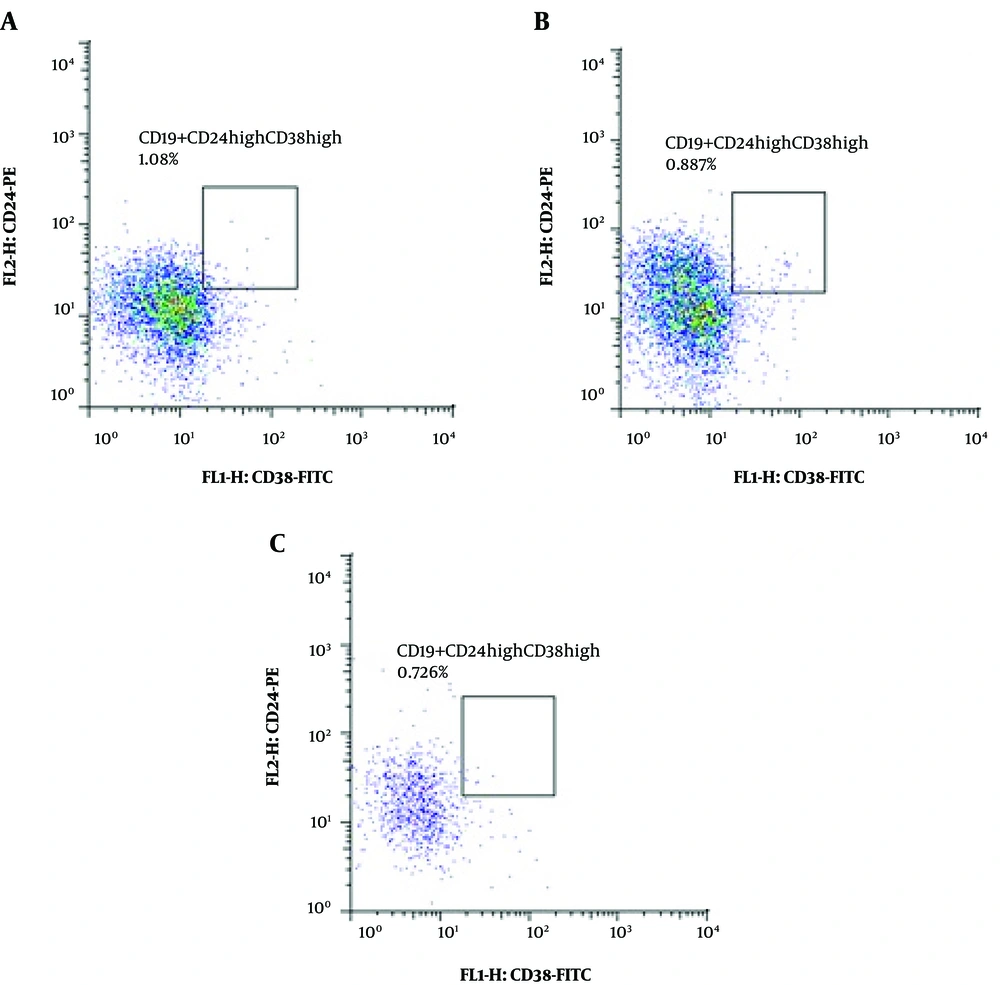
The subset of CD19+CD24highCD38high B lymphocytes (regulatory B cells (Breg)) showed a significant difference between the groups. The percentage of this cell population was significantly higher in healthy controls than in the two other groups (standard-treatment vs. control: P value < 0.0001 and pre-rituximab vs. control: P value = 0.006). However, it decreased significantly after receiving rituximab (pre-rituximab vs. post-rituximab: P value = 0.009) (Figures 5 and 6).
CD19+CD24highCD38high cells frequency (percentage in CD19+ cells) in four groups of standard treatment (steroid + immunosuppressive), pre-rituximab (steroid + immunosuppressive + rituximab), post-rituximab (two months after RTX administration), and healthy controls; *P < 0.05 **P < 0.001 ***P < 0.0001
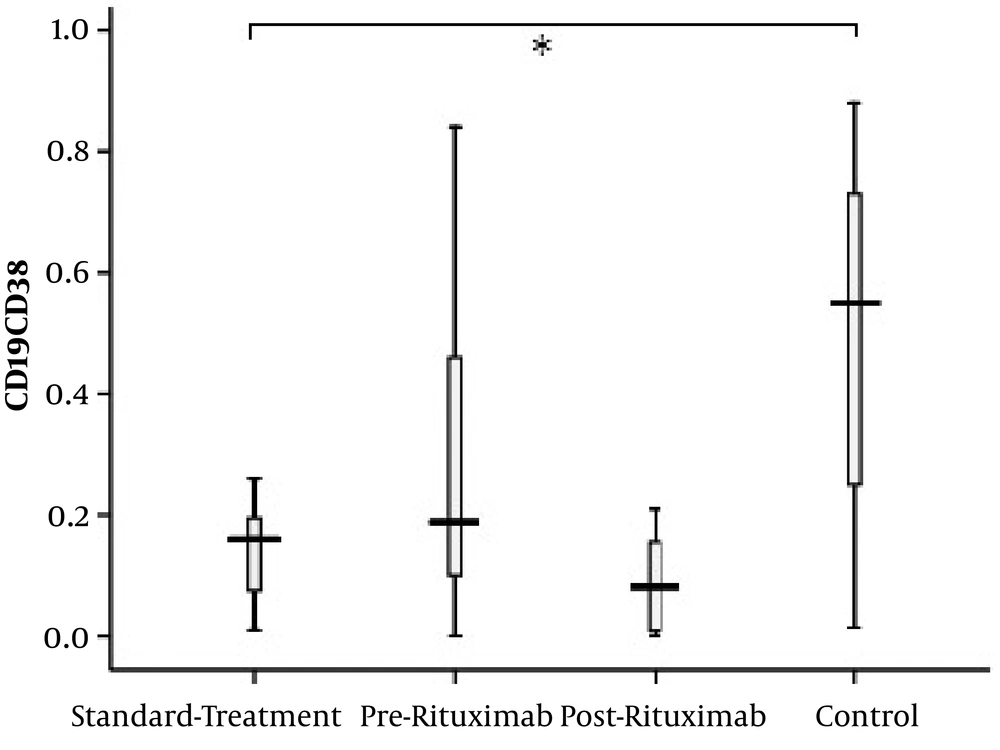
The subset of CD19+CD24-CD38+ B lymphocytes, a class of naïve/mature lymphocytes with normal function, was significantly higher in the control group than in standard treatment patients (P value = 0.01). However, no statistically significant difference was found in CD19+CD24-CD38+ B lymphocytes between the rituximab and control groups nor between pre-rituximab and post-rituximab patients (Figure 7).
CD19+CD24-CD38+ cells frequency (percentage in CD19+ lymphocytes) in four groups of standard treatment (steroid + immunosuppressive), pre-rituximab (steroid + immunosuppressive + rituximab), post-rituximab (two months after RTX administration), and healthy controls; *P < 0.05 **P < 0.001 ***P < 0.0001
5. Discussion
B lymphocytes are produced and developed continuously by lymphoid progenitors in the bone marrow; however, their final differentiation occurs in secondary lymphoid organs, e.g. lymph nodes and spleen (17). Most circulating B cells express CD20 markers; therefore, they are the targets for rituximab, a monoclonal anti-CD20 antibody. Since this antibody is used to treat resistant forms of PMN and causes a considerable decrease in B lymphocyte count, it is essential to identify the most involved B cell subsets to target them specifically and avoid excessive unnecessary immunosuppression. In the present study, peripheral blood B cell subsets were compared between two groups of PMN patients on standard treatment and rituximab treatment and a healthy group. According to our results, the percentage of CD19+ cells, representing the total B lymphocyte population and memory B lymphocytes identified as CD19+CD24+CD38- cells (18, 19) did not show any significant difference between the study groups; however, the percentages of CD19+CD24-CD38+ (naïve/mature B) and CD19+CD24highCD38high (regulatory B) cells were different between PMN patients and healthy individuals.
The CD19+ B cell count was studied by Printza et al. that showed the increased population of these cells in peripheral blood of children with active steroid-sensitive NS (20). However, Colucci et al. did not find any significant difference in total CD19+ and memory B cell population between frequently-relapsing or steroid-dependent NS patients and healthy controls (19). Nonetheless, later they reported higher total, transitional, and memory B cell percentages in steroid-sensitive patients at disease onset before starting immunosuppressive drugs; moreover, the memory B cell count was significantly higher than healthy controls during relapse whereas it was normal in patients during remission (21). Yildiz et al. showed the elevated level of soluble CD19 in the peripheral blood of steroid-sensitive NS patients (22). On the other hand, Kemper et al. found lower levels of CD19+ B cells in untreated relapse cases than in healthy individuals even though their proportion was higher in steroid-dependent patients relapsing on alternate-day steroid (23). In addition, Sellier-Leclerc et al. demonstrated that rituximab efficacy in the treatment of steroid-dependent primary NS children had a direct correlation with CD19+ cell repopulation delay, indicating the implication of these cells in disease pathogenesis (24). Although in our study, the mean percentage of CD19+ cells was higher in PMN patients than in healthy controls, this difference did not reach statistical significance. In addition, the memory B cell count pre and post-rituximab did not alter as expected. Therefore, these cells either are partially resistant to depletion by rituximab or repopulate rapidly after rituximab administration. Nonetheless, regarding the controversial findings concerning CD19+ and memory cells alteration in NS, their percentages at disease onset, remission, and relapse phases require further studies.
Mature/naive B cells characterized by surface markers CD19+CD24-CD38+ are a group of cells recently come out of the bone marrow and are supposed to be activated and proliferated in response to the eligible stimulating foreign antigens, thus constituting a large number of the peripheral B cell population (19). Duddy et al. studying an autoimmune disease model demonstrated that interleukin-10 (IL-10) is markedly produced by naïve/mature B cells while the proinflammatory cytokines lymphotoxin and TNF-α are mainly secreted by memory B lymphocytes (25). In the present study, the mature/naive B cells count in the peripheral blood showed to be significantly lower in standard treatment patients than in healthy individuals. Similarly, Leandro et al. showed that eight months after receiving rituximab, RA patients showed normal B cells reconstitution, with the dominance of naive mature and immature B cells; however, in relapsing patients, repopulation was predominantly the subject of memory B lymphocytes (26). In addition, Melet et al. reported a significant association between the increased number of memory B cells at repopulation and rituximab non-responsiveness (13). It seems that naïve/mature B cells, if act normally and secrete IL-10, might play a protective role against PMN whereas an increase in memory B cells (probably including auto-reactive memory cells) could be assumed as a sign of disease activity.
B regulatory cells (Breg) constitute another subset of B lymphocytes, a considerable proportion of which are characterized by excessive expression of surface markers CD24 and CD38. They are primarily identified within the transitional B cell compartment and are supposed to protect against autoimmune disease such as systemic lupus erythematous (27) and multiple sclerosis (25), as well as renal allograft rejection (28). These cells have shown to suppress CD4+CD25- T cells proliferation and inhibit interferon-γ and tumor necrosis factor-α secretion, partly mediated by IL-10 production. Breg cells also inhibit naïve CD4+CD25- T cell differentiation into T helper 1 (Th1) and Th17 cells and convert them into regulatory T cells (Tregs) (29). Colucci et al. demonstrated that steroid-dependent NS patients had a notably lower percentage of CD38highCD24high and mature B cells (CD38intermediateCD24low) than healthy controls (19). Similarly, our study showed higher Breg cell percentage in the healthy group than in both groups of standard treatment and rituximab treatment patients. Regarding the implication of these cells in regulating immune responses, their insufficiency might result in the elevated risk of autoimmunity.
In summary, despite the limited number of studies concerning B lymphocytes implication in NS, as the most prominent finding, they showed an increased percentage of memory B cells during disease activity. Our study indicated that the number of regulatory B cells decreased in both standard treatment and rituximab-receiving PMN patients and the proportion of naïve/mature B lymphocytes was lower in the former group. Moreover, the memory B cells count did not reduce significantly two months after rituximab administration. Hence, it might be the best choice to target the memory B cell subset in immunosuppressive therapy while avoiding the Breg or naïve/mature B lymphocyte depletion to obtain more favorable sustained outcomes. Cell therapy using Breg transfer into PMN patients could be another alternative that remains to investigate in the future.