1. Background
Urinary tract infections (UTIs) as one of the most important infections in children can result in several complications. Three major forms of UTIs include pyelonephritis, cystitis and asymptomatic bacteriuria (1, 2). Acute pyelonephritis is one of the most common diseases of bacterial infections in children, and scarring in the kidney is one of the long-term complications in these patients. Escherichia coli is often known as the cause of pyelonephritis, however other organisms, including Klebsiella, Enterococcus, Enterobacter, Proteus and Pseudomonas are also involved (3, 4).
Clinical manifestations of the acute pyelonephritis can be very non-specific, which makes it difficult to diagnose. Sometimes, the only sign in a child with acute pyelonephritis is an asymptomatic jaundice (1). In children aged 2 to 5 years, the symptoms and complaints of pyelonephritis may be non-specific with no complaints of the urinary system, and also the patient may only report fever and abdominal pain. Manifestations are more classic in children at the age of five years, which include dysuria, urinary frequency, urinary urgency, fever, pain and hematuria (5).
Although treatment of UTI is usually simple, however with an increase in drug resistance worldwide, the choice of treatment in children with UTIs should be based on the therapeutic types of resistant treatments. The benefits of antibiotic prophylaxis have discussed following the first infection due to UTI in a child with vesicoureteral reflux (6). Outpatients with oral fluoroquinolone antibiotics are successfully treated and this therapeutic method has shown effective in most patients with uncomplicated pyelonephritis. Therapy for inpatients includes administrating intravenous antibiotics, such as fluoroquinolone, aminoglycoside with or without ampicillin, or third-generation cephalosporins and also the standard treatment period is 14 days (7).
In addition to antibiotic therapy, other therapies can be used to control the clinical manifestations of the patients and complications of UTIs, which has been studied in a limited number of studies (8).
2. Objectives
Based on the mentioned issues as well as the importance of complications of pyelonephritis, this study aimed to investigating effect of montelukast on the pyelonephritis symptoms in children.
3. Methods
3.1. Study Setting
This study was conducted in the pediatrics clinic of Amir-Kabir Hospital, Arak, Iran.
3.2. Study Population
This clinical trial was conducted on 100 children with clinical manifestations of pyelonephritis. The patients were divided into two groups (n = 50 per group), including the intervention and control groups.
3.3. Measurement
Patients in both groups received routine antibiotic therapy and for the intervention group montelukast (5 mg/day, oral) was also administrated for 14 days. Finally, the clinical manifestations of the patients were evaluated and compared between two groups.
3.4. Ethical Considerations
All patients and their parents were informed about the study objectives prior to the study and also the informed consent was obtained. The patients younger than 12 years were enrolled and they were allowed to withdraw from the study at any time. The research team was required to observe all the Helsinki Statement as well as the ethics considerations of the Arak University of Medical Sciences, (ethical code: IR.ARAKMU.REC.1397.304; IRCT code: IRCT20190303042893N1).
3.5. Statistical Analysis
After collecting data, descriptive statistical methods were used to determine the frequency of variables. We used mean and standard deviation for analyzing quantitative variables, and Student t-test for qualitative variables. The P values of less than 0.05 were considered as significant.
4. Results
The mean age (± SD) of the intervention and control groups was 7.2 (± 0.43) and 7.18 (± 0.43) years, respectively with the age range of 3 - 14 years. In addition, 38 and 12 cases in the intervention group were female and male, and in the control group, 34 and 16 cases were female and male, respectively (Table 1).
| Variables | Groups | P Value | |
|---|---|---|---|
| Control | Intervention | ||
| Age, mean ± SD | 7.18 ± 0.43 | 7.2 ± 0.43 | 0.9 |
| Gender, No. (%) | 0.07 | ||
| Male | 12 (24) | 38 (76) | |
| Female | 16 (32) | 34 (68) | |
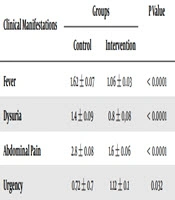
Moreover, the results showed that the mean duration (day) of clinical manifestations of pyelonephritis were significantly lower in the intervention group than the control group, which included duration of fever (P < 0.0001), dysuria (P < 0.0001), abdominal pain (P < 0.0001) and urgency (P = 0.003) (Table 2).
| Clinical Manifestations | Groups | P Value | |
|---|---|---|---|
| Control | Intervention | ||
| Fever | 1.62 ± 0.07 | 1.06 ± 0.03 | < 0.0001 |
| Dysuria | 1.4 ± 0.09 | 0.8 ± 0.08 | < 0.0001 |
| Abdominal Pain | 2.8 ± 0.08 | 1.6 ± 0.06 | < 0.0001 |
| Urgency | 1.12 ± 0.1 | 0.72 ± 0.7 | 0.032 |
aValues are expressed as mean ± SD.
5. Discussion
In this study, it was observed that the length of clinical manifestations of pyelonephritis in interventional group was lower compared to the control group. However, various results have been reported other studies.
Abdel-Raheem and Khedr in a study, evaluated the montelukast effect on supporting the kidneys in the patients who consumed methotrexate and concluded that montelukast reduced the effects of methotrexate on the kidneys (9).
These results are consistent with our findings indicating the beneficial effects of this drug in treating patients. Otunctemur et al. (10) also compared the effect of montelukast on the reduction of renal complications with gentamicin and stress. They compared the interactions between these two drugs in the experimental mice and concluded that montelukast prevented resulted complications (10). These results are also consistent with the results of the present study. On the other hand, Bisgaard et al. also studied the effect of montelukast on the treatment of respiratory viral infection and reported that montelukast is an inhibitor of respiratory viral infection (11). This is also consistent with the results of the current assessment representing the efficacy of montelukast in reducing inflammation. Han et al. evaluated the anti-inflammatory effect of montelukast and concluded this drug acts as an anti-inflammatory agent in these patients (12), which is in line with our results. Ahanchian et al. also evaluated the effect of montelukast on inflammation and viral infections. They stated that montelukast is an inhibitor of inflammation and asthma (13). In addition, Huang et al. also assessed the effect of Ibuprofen on clinical manifestations and transplantation of renal scars in pyelonephritis and observed that renal scarring in this group that consumed the antibiotics alone was not significantly reduced (14), which is also consistent with our findings.
Moreover, Huang et al. (14) examined the diagnostic use of procalcitonin in pyelonephritis and mentioned that serum procalcitonin can be a sensitive marker for early detection of acute pyelonephritis, although its disadvantages cannot be detected (15). This study also has demonstrated the efficacy of procalcitonin in detection and controlling the symptoms of these patients. In addition, Tagvaie et al. study on the therapeutic effect of ciprofloxacin and ceftriaxone has shown that the use of oral ciprofloxacin has been effective in the treatment of uncomplicated acute pyelonephritis in women (16), which is consistent with the results of our evaluation. Accordingly, the importance of using anti-inflammatory drugs, such as montelukast in the treatment of patients with pyelonephritis has be emphasized.
5.1. Conclusions
Based on the obtained results as well as the result of other relevant studies, montelukast can lead to a rapid improvement in clinical manifestations in children with pyelonephritis and it can be used as an effective auxiliary treatment in these patients.