1. Background
Chronic kidney disease (CKD) is increasing in prevalence in India and globally (1, 2). This is attributed to the increased incidence of diabetes, hypertension, and lifestyle-related factors. Raised awareness and better accessibility to health care facilities have also contributed to this high prevalence. Hemodialysis is still the predominant form of renal replacement therapy in India and many other countries (1). The patient population on dialysis is vulnerable to many complications and premature mortality due to disease per se, accelerated atherosclerosis, or dialysis treatment itself. Cardiac problems comprise major causes of mortality in dialysis patients and it has received more attention. Stroke is also a leading cause of mortality and morbidity in this dialysis population. Various studies have estimated the risk of stroke at 5 - 30 times the general population, with some showing a preponderance of ischemic stroke and some others of hemorrhagic stroke. Most data on stroke in dialysis come from Japan and the United States and the Indian data are sparse.
2. Objectives
The present study focused on intracranial bleeding (ICB) in dialysis patients by analyzing the clinical profile, various associated risk factors, and the impact of ICB on short-term patient survival.
3. Methods
This case-control study was undertaken at St. John Medical College, Bangalore, India, over a span of three years. This tertiary care hospital caters to people across all socioeconomic strata. The study included patients with CKD on long-term maintenance hemodialysis at St. Johns who either suffered ICB in the hospital or came to the emergency with the event. Those with ischemic stroke were excluded. We recorded clinical features such as vital signs, Glasgow coma score, comorbid diseases, viral serology, hemogram, blood biochemistry including liver and renal panel, and coagulation profile. The CT/MRI reports were noted for the site of ICB, the size of bleeding, and intraventricular extension. Dialysis records were analyzed for blood pressure readings seven and 30 days before the event, as well as laboratory reports. Thus, 30 cases with ICB were identified during the study period. The cases were well matched to the control population (n = 60) for demographics and duration of dialysis. Data on the controls were collected from dialysis records. Ethical committee clearance was obtained.
Summary statistics were done by proportion, mean, median, SD, and IQR. Inferential statistics were done by the chi-square test, independent t test, and Mann-Whitney test. All measurements were done using SPSS 16.0.
4. Results
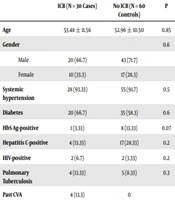
Males outnumbered females in our study (69.5% vs. 30.5%). The average age of the study population was 53.48 years. Moreover, 92.7% of the study population was hypertensive (cases 95.7%, controls 91.3%) and 60.87% of the study population was diabetic (cases 65.2%, controls 58.7%) (Table 1).
| ICB (N = 30 Cases) | No ICB (N = 60 Controls) | P | |
|---|---|---|---|
| Age | 53.48 ± 11.56 | 52.96 ± 10.50 | 0.85 |
| Gender | 0.6 | ||
| Male | 20 (66.7) | 43 (71.7) | |
| Female | 10 (33.3) | 17 (28.3) | |
| Systemic hypertension | 28 (93.33) | 55 (91.7) | 0.5 |
| Diabetes | 20 (66.7) | 35 (58.3) | 0.6 |
| HbS Ag-positive | 1 (3.33) | 8 (13.33) | 0.07 |
| Hepatitis C-positive | 4 (13.33) | 17 (28.33) | 0.2 |
| HIV-positive | 2 (6.7) | 2 (3.33) | 0.2 |
| Pulmonary Tuberculosis | 4 (13.33) | 5 (8.33) | 0.3 |
| Past CVA | 4 (13.3) | 0 | |
| Frequency of dialysis | 0.06 | ||
| Two per week | 8 (26.7) | 30 (50) | |
| Three per week | 22 (73.33) | 30 (50) | |
| Heparin (anticoagulation during dialysis) | 0.06 | ||
| Systemic heparin | 2 (6.7) | 2 (3.3) | |
| Tight heparin | 26 (86.7) | 58 (96.7) | |
| Low-molecular weight heparin | 0 (0) | 0 (0) | |
| Saline | 2 (6.7) | 0 (0) | |
| Heparin average Dose | 90.36 ± 24.8 | 86.48 ± 12.9 | 0.5 |
| Major causes of CKD | 0.09 | ||
| Diabetic nephropathy | 17 (56.7) | 39 (65.0) | |
| Chronic glomerular | 11 (36.7) | 13 (21.7) | |
| Chronic interstitial disease | 2 (6.7) | 5 (8.33) | |
| Chronic allograft nephropathy | 0 (0) | 3 (5) | |
| Platelet Count (lakhs/microliter) | 17738 ± 807274 | 217570 ± 73324 | 0.06 |
| Aspirin Use | 5 (16.7) | 17 (28.3) | 0.7 |
| Clopidogrel Use | 6 (20.0) | 8 (13.3) | 0.7 |
| Aspirin + clopidogrel | 4 (13.3) | 12 (20.0) | 0.7 |
| No antiplatelets | 15 (50) | 23 (38.33) | 0.7 |
aValues are expressed as No. (%) or mean ± SD.
Diabetes was the most common cause of CKD, accounting for 62.3% (cases 56.5%, controls 65.2%), followed by chronic glomerulonephritis (CGN) (Table 1). An average of 28,527 dialysis sessions is conducted annually at our center; 720 patients were on maintenance hemodialysis during the study period, of whom 30 suffered ICB (prevalence 3.19%).
About 57.8% (52/90 patients) of our study patients were on thrice-weekly dialysis and the rest was on twice a week dialysis (38/90 patients). All patients underwent dialysis sessions for four hours, with a P value of 0.07 for the frequency of dialysis between cases and controls (Table 1). In patients with ICB, the mean duration of dialysis was 21.78 months with a range of 1 - 72 months (cases and controls were matched for the duration of dialysis).
The average dose of unfractionated heparin used was 90.36 units per kg body weight in cases and 86.48 units per kg among controls (P = 0.5). Fifteen (out of 28) patients with ICB were using antiplatelet at the time of the event (P = 0.6). Prothrombin time (PT) and activated partial thromboplastin time (APTT) done after the event were not deranged in cases. Platelet counts were available in both groups and were not different between the two groups (Table 1). The BP recordings at the time of stroke (current) and BP readings in the preceding one week and one month were analyzed, which showed no statistically significant difference (Table 2).
| ICB (Cases) | No ICB (Controls) | P | |
|---|---|---|---|
| Mid dialysis SBP, mmHg (on the day of the event or in the last HD before the event) | 148.57 ± 19.82 | 146.30 ± 18.1 | 0.7 |
| Mid DBP Dialysis, mmHg (on the day of the event or in the last HD before the event) | 84.29 ± 7.46 | 84.35 ± 6.2 | 0.9 |
| Pre-dialysis SBP, mmHg (on the day of the event or in the last HD before the event) | 145.45 ± 16.83 | 143.9 ± 17.1 | 0.7 |
| Pre-dialysis DBP, mmHg (on the day of the event or in the last HD before the event) | 84.36 ± 7.03 | 84.6 ± 6.4 | 0.9 |
| Seven-day prior SBP, mmHg | 151.74 ± 18.25 | 143.95 ± 17.9 | 0.1 |
| Seven-day prior DBP, mmHg | 87.65 ± 7.95 | 85.58 ± 5.5 | 0.2 |
| 30-day Prior SBP, mmHg | 146.36 ± 13.99 | 141.46 ± 17.1 | 0.8 |
| 30-day Prior DBP, mmHg | 85.91 ± 7.34 | 85.54 ± 5.9 | 0.2 |
aValues are expressed as mean ± SD.
Serum albumin at the time of stroke and prior albumin values were significantly lower in those with ICB than in controls (2.65 vs. 3.15, P = 0.005 at the event; 2.70 vs. 3.07, P = 0.036 prior to the event). Serum sodium, potassium, calcium, phosphorous, and liver function tests were not different between cases and controls. Random blood glucose levels (RBS) were slightly high in cases (P = 0.04) (Table 3).
| Cases | Controls | P | |
|---|---|---|---|
| Serum Protein current | 6.51 ± 1.11 | 7.00 ± 1.03 | 0.08 |
| Serum protein prior | 6.63 ± 0.80 | 6.77 ± 0.93 | 0.5 |
| Albumin current | 2.65 ± 0.60 | 3.15 ± 0.7 | 0.005 |
| Albumin prior | 2.70 ± 0.62 | 3.07 ± 0.7 | 0.036 |
| B urea current | 103 (60 - 132) | 80 (57 - 119) | 0.6 |
| B urea prior | 112 (60 - 132) | 124 (75 - 147) | 0.7 |
| Na current | 135.09 ± 3.9 | 135.00 ± 4.7 | 0.5 |
| Na prior | 133.8 ± 5.65 | 135.60 ± 3.7 | 0.2 |
| K+ current | 4.56 ± 0.99 | 4.82 ± 0.9 | 0.9 |
| K+ prior | 4.71 ± 0.63 | 4.83 ± 0.9 | 0.1 |
| Calcium current | 8.33 ± 1.58 | 8.53 ± 0.7 | 0.3 |
| Calcium prior | 8.67 ± 1.13 | 8.34 ± 0.8 | 0.6 |
| Phosphorous current | 4.3 (2.7 - 5.7) | 4.4 (3.4 - 5.3) | 0.3 |
| Phosphorous prior | 4 (3.1 - 4.8) | 4.9 (3.9 - 6.4) | 0.8 |
| Random blood sugar current | 180.70 ± 73.56 | 142.03 ± 69.9 | 0.04 |
| HB% current | 9.38 ± 1.79 | 9.37 ± 1.7 | 0.9 |
| HB% prior | 9.03 ± 1.81 | 9.66 ± 1.6 | 0.1 |
| AST current | 30 (20 - 55) | 24 (16 - 39.5) | 0.9 |
| AST prior | 20 (18 - 28) | 21.5 (14.5 - 30) | 0.09 |
| ALT current | 35 (22 - 64) | 33.5 (24 - 54.5) | 0.9 |
| ALT prior | 29 (24 - 60) | 29.5 (24 - 44.5) | 0.8 |
aValues are expressed as median (IQR) or mean ± SD.
the most common site of bleeding was intraparenchymal (60%) followed by Sub dural hematoma (30%). Three patients had primary intraventricular bleeding. Clinical features like vomiting, altered sensorium, lateralizing signs, and seizures could not predict the site of ICB. The mean time to occurrence of ICB event was 2 hours from last heparin dose (range 0 - 24 hours; five patients had ICB while dialysis was in process, the rest had it after dialysis was completed). There was a history of trauma in 30% of the cases, old CVA in 21.7%, evidence of micro bleeding in 14.3%, and lacunar infarcts in 9.5% (Table 4).
| Values | |
|---|---|
| Number of ICB cases presenting with vomiting | 20 (65.2) |
| Number of ICB cases presenting with altered sensorium | 20 (65.2) |
| Number of ICB cases with lateralizing deficits | 11 (35.0) |
| Number of ICB cases with seizures | 3 (10) |
| GCS on admission | 7 (3 - 14) |
| Site of bleeding | |
| Intraparenchymal | 18 (60) |
| Subdural | 9 (30) |
| Subarachnoid hemorrhage | 3 (10) |
| Past history of CVA | 4 (13.33) |
| Past history of IHD | 3 (10.0) |
| Time to event from last heparin dose, h | 2 (0 - 24) |
| Micro bleed (yes) | 4 (13.33) |
| Lacunar infarct (yes) | 3 (10.0) |
| Number of ICB patients on erythropoietin | 8 (26.7) |
| Trauma history | 9 (30.4) |
| Outcome of ICB by day 30b | |
| Complete recovery to normal (n = 0) | |
| Partial recovery (n = 7) | 14 (10 - 15) |
| Death (n = 23) | 3.5 (3 - 13) |
Abbreviations: CVA, cerebrovascular accident; IHD, ischemic heart disease.
aValues are expressed as No. (%) or median (IQR).
bP = 0.1 in Mann-Whitney test.
There were 23 deaths in the first one month and the cause was probably brain stem dysfunction and herniation; two patients succumbed to septic shock.
5. Discussion
The prevalence of CKD is increasing in India and globally. All stages of CKD are associated with high morbidity and mortality. This risk, which begins in early CKD, increases as CKD advances (3). Coronary artery disease and stroke are the leading causes of mortality in the chronic dialysis population. The incidence of stroke in dialysis patients varies from 10 to 33 per 1000 patient-years and is 5 - 30 times greater than in the general population. The age-adjusted relative risk of stroke among dialysis patients compared to the general population was 6.1 for Caucasians and 9.7 for African-American males (4). Patients on dialysis had a higher incidence of hemorrhagic stroke than the general population. A 22-year single-center study of stroke in dialysis patients noticed that stroke occurred at a younger age (64 ± 10 vs. 67 ± 13 years) and hemorrhagic stroke was more common (52%) than ischemic stroke (4, 5).
Risk factors for stroke include modifiable and non-modifiable factors such as the old age, non-Caucasian and Asian ethnicity, and family history. Diabetes and hypertension have emerged as major risk factors for ischemic and hemorrhagic stroke (6). Chronic kidney disease has a higher burden of traditional risk factors and nontraditional risk factors like hyper-homocysteinemia, hyperuricemia, chronic inflammation, asymmetric dimethylarginine, oxidative stress, anemia, thrombogenic factors, endothelial dysfunction, arterial stiffness, and impaired cerebral auto regulation (7). Patients with CKD and stroke have shown an increased prevalence of intracranial artery calcification from 76.2 to 95% in various studies (8). Pulse wave velocity, which reflects arterial stiffness, is high in CKD. Vascular calcification and stiffness can worsen hypertension and together with anticoagulation may increase the risk of hemorrhagic stroke (9). Anemia, which invariably occurs as CKD progresses, leads to the increased stroke risk, mostly ischemic stroke (10), but may also result in bigger hematomas as shown by studies of critically ill patients (11) although not much literature is available on the dialysis population.
Dialysis patients with hemorrhagic stroke had poor survival and high mortality in our study, which was 76.7% at one month that is similar to other studies showing 53% - 79% mortality (10-12). Mortality is highest in the first three months and is much higher than the mortality of patients with cerebral infarction (11-13). In our study, most deaths occurred either immediately or within three months of the event. Patients who suffered ICB were on dialysis for a longer duration than those who suffered cerebral infarction; in our study, 39.1% had been on dialysis for three years or more. Patients who present the reduced levels of consciousness on admission have larger hematoma and irregular hematoma and often reveal hematoma enlargement on repeat CT scans (14, 15). Those with large hematomas, especially more than 60 mL, and pontine hematoma showed a poor outcome (16). In the current study, we did not systematically analyze the hematoma volume and its relationship with the outcome. Abnormal coagulation, such as prolonged prothrombin time and fibrin degradation products, is seen in some studies (17) whereas no significant correlation was seen between fibrinogen levels and hematoma size in others (18). In our study, there was no significant difference in the coagulation profile as made out by platelet counts within the normal range, APTT, PT, bleeding time, and clotting time. Some studies reported the most common site of bleeding in dialysis patients is the basal ganglia and ventricles (19); in our study, it was intraparenchymal, followed by subdural hematoma.
Hypertension is a risk factor for ICB. In CKD, blood pressure (BP) is difficult to control and often requires multiple antihypertensive medications. Poorly controlled BP after an ICB event can increase the risk of re-bleeding and worsen the neurological outcomes. The strict control of BP is required to reduce the size of intracerebral bleeding and improve the prognosis following a bleeding event (16-18). What correlates better with the outcome, initial BP or later BP recordings, systolic BP or diastolic BP, is subject to debate. Kim et al.’s (20) study found no relationship with the initial BP but the third day BP was correlated with outcome. In our study, we did not observe a statistically significant difference in current BP or prior BP and the BP of the case group was not different from that of the selected control group. This does not undermine the importance of BP control; however, many of our patients were on multiple antihypertensive medications. The most common cause of death in ICB patients is brain stem herniation, which is the result of raised intracranial tension due to either hematoma per se or cerebral edema caused by dialysis disequilibrium as a result of rapid changes in osmolality with intermittent therapies (21).
Malnutrition is a well-recognized problem in hemodialysis patients. Its causes are multifactorial, including increased common catabolism and decreased intake (12, 17). They are due to the increased levels of pro-inflammatory cytokines in CKD and dialysis patients. The malnutrition inflammation complex leads to poor outcomes and increases all-cause mortality (22). Under-nutrition is associated with a higher risk of ICB in dialysis patients. This is in contrast to the general population where obesity confers a higher stroke risk. Low albumin affecting erythrocyte deformability and endothelial dysfunction may well explain the increased stroke risk in malnourished patients (23, 24). The observation of low albumin in our study concurs with the available literature and emphasizes the importance of maintaining good nutrition in dialysis patients. The control group without ICB had higher serum albumin levels.
Heparin is the most commonly used anticoagulant for hemodialysis (25). It is used as an initial bolus, followed by hourly boluses, with the last dose given one hour prior to the termination of dialysis. Although it potentially causes serious bleeding complications, in view of its short half-life of 30 minutes to two hours, bleeding episodes related to intracranial, gastrointestinal and pericardium hemorrhages are not common (26). The safety of low-molecular-weight heparin has been proven by many studies though its anticoagulant effect lasts four hours (27, 28). Heparin on a background of uremic bleeding diathesis could lead to an increased risk of hemorrhagic stroke (29). If intracerebral bleeding does occur in a dialysis patient, systemic heparinization can increase the hematoma size (30) and worsen the outcome. The use of aspirin was associated with increased intracerebral bleeding in a study and did not confer significant benefit to cardiovascular outcome (31). Our current study showed no significant difference in the average heparin dose and antiplatelet use between the case and control groups.
Diabetes may increase the vascular risk but its role is not clear in the etiopathogenesis of spontaneous intracranial hemorrhage, with some studies showing an positive association, others not, and even some studies showing a reduced incidence of bleeding in the general population (32-34). Poor glycemic control confers increased mortality in hospitalized patients. Our study patients had higher blood sugar at the time of the event than the controls.
In conclusion, hemodialysis patients have multiple risk factors for ICB. Blood pressure was high during the event although it did not achieve statistical significance. Optimal control of BP is important to reduce ICB and its complications. The common site of bleeding was intraparenchymal, followed by SDH. There was a higher incidence of ICB, although not statistically significant, in patients on thrice-weekly dialysis. Low serum albumin, which is an indicator of poor nutritional status, carried a higher risk for ICB; thus, it is important to maintain a good nutrition status in dialysis patients. Patients with ICB had high mortality and most deaths (76.7%) occurred within one month of the event.
One of the main limitations of our study is the sample size. Larger sample size would have better addressed the role of many factors including uncontrolled glycemic state, hypertension, and thrice-weekly dialysis in increased predisposition to ICB in the dialysis population.