1. Background
Central nervous system (CNS) neoplasms are among the most prevalent cancers, especially in adults (1). The overall incidence of these tumors in Iran was 2.74 per 100,000 person-year, with a benign to malignant ratio of 1.07 during the 2010s (2). Symptoms vary based on the location of tumors, however, focal neurologic deficit, seizure, and headache are frequently reported symptoms (3). The initial involvement of patients with CNS tumors routinely consists of magnetic resonance imaging (T1, T2-weighted studies and fluid-attenuated inversion recovery sequences) (3, 4).
Even with a characteristic imaging appearance of neoplastic lesions, histologic assessment and confirmation is essential to determine biology, grade, and genetic alternations of the tumor (5). However, in some conditions such as brain stem gliomas, diagnosis is generally based on the clinical presentation and imaging findings because of the substantial risk of mortality and morbidity of tissue sampling in such cases (3).
There are some major difficulties in pathological diagnosis of the brain tumors. With the introduction of the newer techniques of microsurgical resection in the context of brain tumors, nowadays pathologists are dealing with smaller tissue samples. This issue causes difficulties in the definitive histologic diagnosis. In a report by Chandrasoma et al. the correlation between the stereotactic and resection diagnoses was precise in just 19 of 30 cases (6). In an another study by Jackson et al., at the University of Texas M.D. Anderson cancer center, the discrepancy in diagnosis of glial tumors existed in 49% of patients, which is likely to affect the prognosis in 38% of patients and treatment in 26%. However, the current study reduced the discrepancy to 38% by reviewing the specimens (7).
Another difficulty is the significant interobserver variation in pathologic assessment of brain tumors. In the studies by Scott et al. and Hildebrand et al. authors noted a high degree of discordance in pathologic diagnosis of brain tumors, especially in the diagnosis of astrocytomas (8, 9). In a review by van den Ben entitled “Interobserver Variation of the Histopathological Diagnosis in Clinical Trials on Glioma: A Clinician’s Perspective”, the interobserver variation in the pathological diagnosis was considered as a well-recognized and major issue both in the management and diagnostic confirmation of patients with brain tumors (10).
Because of these problems, studies of the recent decades focused on the genetic basis of brain tumors (11). Recently, world health organization (WHO) revised the CNS tumors classification according to the genetic features (12). Assessment of mutations in isocitrate dehydrogenase (13) and 1p/19q codeletion (14) are among the most widely used genetic tests, which demonstrate prognosis and significantly predict response to treatment. However, these tests are very expensive and often unavailable in the developing countries and in some situations cannot help a decision between the differential diagnoses.
Considering the importance of definite histologic diagnosis of brain tumors and limitations of performing such genetic tests in Iran, using other diagnostic features of brain tumors such as imaging is critical to the accurate diagnosis. Therefore, the current study aimed at evaluating the discordance between imaging data and pathologic diagnosis of patients with brain tumors.
2. Methods
The current descriptive study retrospectively assessed the medical documents of patients referred to neuro-oncology clinics of Omid and Emam Reza hospitals affiliated to Mashhad University of Medical Sciences, Mashhad, Iran from 2009 to 2010. A checklist was provided for data collection. The study employed the imaging and pathologic criteria presented by Gondi et al. in the Perez and Brady’s principles and practice of radiation oncology (3). Since the glial tumors and meningioma are the majority of brain tumors, the study just reviewed their imaging and pathologic features briefly. Most of the high-grade gliomas usually show a vasogenic edema and ring enhancement around central necrotic regions in the imaging studies. These features typically occur in glioblastoma multiform (GBM); however, anaplastic gliomas may not enhance contrast media. Low-grade gliomas include pilocytic and non-pilocytic astrocytoma, from which the pilocytic type is a well-defined tumor, consisted of cystic and solid components. The non-pilocytic type usually presents as an ill-defined, diffuse, and non-enhancing tumor. Meningiomas usually present as an enhancing lesion typically located at cerebral convexities, falx cerebri, tentorium cerebelli, cerebellopontine angle, and sphenoidal ridge.
For the pathologic evaluation of astrocytomas, presence of any of the 3 findings of nuclear atypia, mitotic activity, vascular proliferation and necrosis were considered as GBM. The histopathologic features of anaplastic gliomas included increased cellularity, nuclear atypia, and marked mitotic activity, without necrosis or neovascularization. The histopathologic features of meningiomas include bland and whorled cells forming psammoma bodies. All medical documents including pathologic reports and imaging studies were reviewed by an expert neuro-oncologist. In case of discordance between pathologic and imaging studies, the slide and block of tumor was reviewed by another pathologist. Data were reported using descriptive tests.
3. Results
Overall, there were 240 documents with the diagnosis of brain tumor. Eleven (4.5%) patients were initially found to have imaging/pathologic discordance. After pathologic review of these 11 patients, diagnosis was changed in 82% of them and the previous diagnosis was confirmed just in 2 patients. The disease in which imaging/pathologic discordance occurred in its diagnosis most frequently was glioblastoma (36.7%) (Table 1). Six out of nine patients whose diagnosis was changed received a completely altered treatment. The current study presented 2 incredibly interesting cases (Table 1) that had opposite outcomes in which management of one of them changed from simple periodic follow-up to a heavy chemoradiation and the other one had the opposite scenario.
| Number | Age | Sex | Presentation Symptoms | Imaging Findings/Interpretation | Imaging Findings were Compatible with | Initial DX | Final DX |
|---|---|---|---|---|---|---|---|
| 1 | 26 | Female | Headache, nausea/vomiting, right hemiparesis | Solid mass with central necrosis, peripheral edema and heterogeneous contrast enhancement, midline shifting | HGG | Meningioma | Anaplastic astrocytoma |
| 2 | 26 | Male | Generalized seizure | Frontal lobe mass, without contrast enhancement | LGG | Glioblastoma multiforme | Glioblastoma multiforme |
| 3 | 52 | Male | Headache, ear pain | Right parietal hypersignal homogenous mass, peripheral edema, entirely contrast enhancement | Meningioma | Anaplastic astrocytoma | Meningioma |
| 4 | 22 | Male | Seizure | Left temporal lobe mass, a low degree of peripheral edema | LGG | Anaplastic astrocytoma | Low grade astrocytoma (grade 2) |
| 5 | 22 | Male | Hemiplegic, headache, nausea, vomiting | Left frontoparietal mass, central necrosis, peripheral edema | GB | Anaplastic astrocytoma | Glioblastoma multiforme |
| 6 | 14 | Male | Headache, nausea, vomiting | Heterogeneous intraventricular mass, contrast enhancement, vasogenic edema | HGG | Medulloblastoma | High grade glioma |
| 7 | 61 | Male | Headache, visual field defect | Mass in sella without edema,sell widening | hypophysis adenoma | Glioblastoma multiforme | Glioblastoma multiforme |
| 8 | 40 | Male | Headache, hemi paresis | Right parietal mass, heterogeneous contrast enhancement, peripheral edema | HGG | Fibrillary astrocytoma (grade 2) | Glioblastoma multiforme |
| 9 | 72 | Female | Drowsiness, Urinary Incontinence, dizziness | Right frontal homogenous mass with peripheral edema | HGG | Angimatous meningioma (grade I) | Atypical meningioma (grade II) |
| 10 | 47 | Male | Left hemiparesis, headache, facial nerve paresis, nausea, vomiting | Right temproparietal heterogeneous mass and peripheral edema | HGG | Meduloblastoma | Glioblastoma multiforme |
| 11 | 15 | Female | Headache | Right temproparietal mass. Contrast enhancement, vasogenic peripheral edema | High grade lesion or Meningioma | Ganglioma (grade 1) | High grade glioma |
Data of Patients with Imaging/Pathologic Discordance
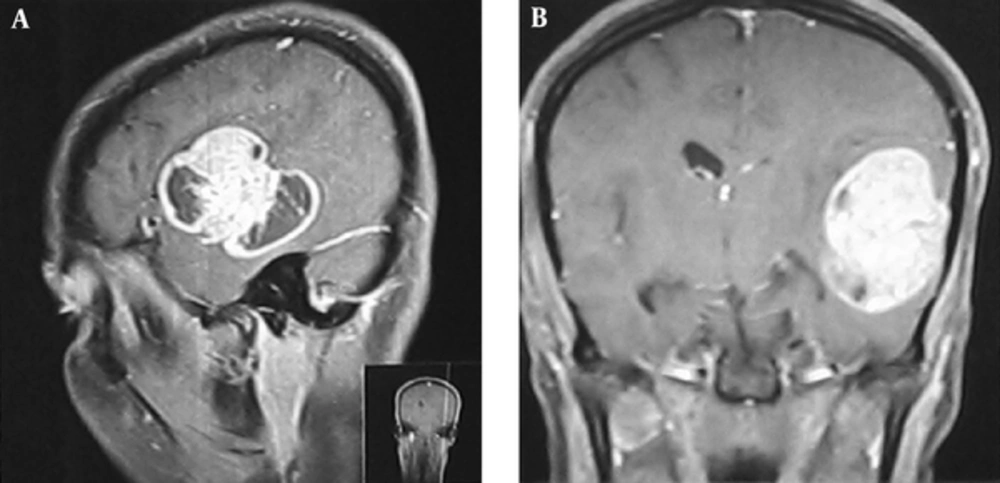
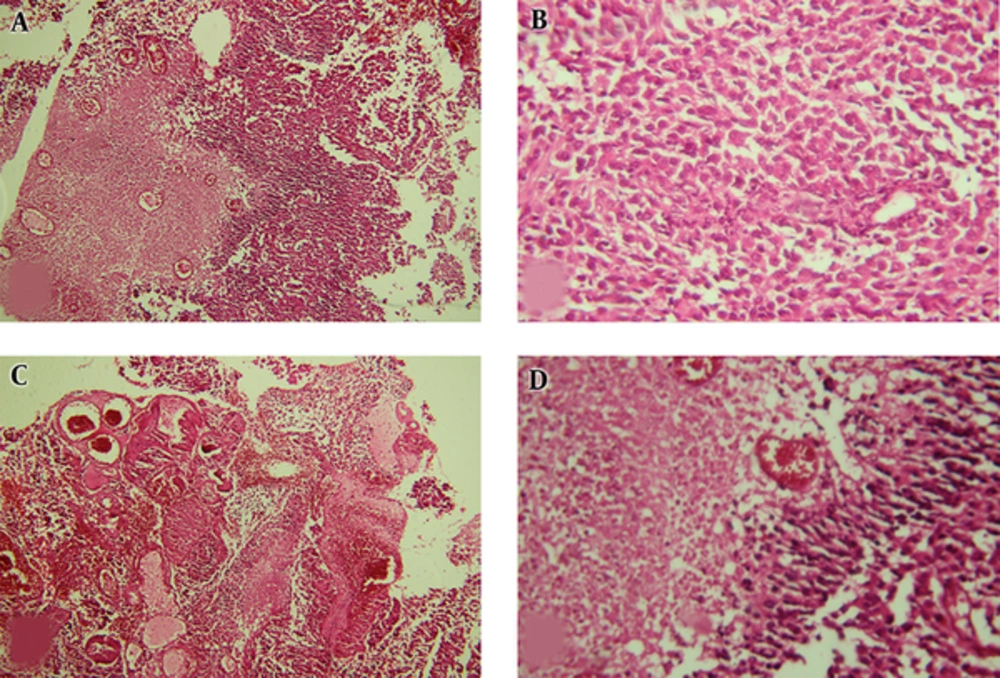
The first case was a 26-year-old female who referred to the neurosurgery department with headache and nausea/vomiting. Physical examination revealed a right hemiparesis. On magnetic resonance imaging (MRI), there was a solid mass with central necrosis, peripheral edema and heterogeneous contrast enhancement that caused midline shifting (Figure 1). All of these findings were consistent with a high grade brain tumor. The initial pathologic diagnosis was meningioma that in most cases is considered as a low grade tumor. Therefore, there was inconsistency between imaging and pathologic findings. Reviewing the pathologic assessments confirmed the diagnosis of anaplastic astrocytoma, which is a high grade tumor (Figure 2). She received standard dose radiotherapy (60Gy) with concurrent and adjuvant chemotherapy. During follow-up, after 3 years, the patient had a local recurrence and underwent re-resection. The pathologic assessment revealed a high-grade astrocytoma. As mentioned previously, if the patient was managed based on the primary diagnosis of meningioma, she could have just been followed up or undergone low-dose radiotherapy with 50 - 54 Gy.
Pathologic Findings of 1st Case; A, Neoplastic Lesion with Diffuse Pattern and Geographic Necrosis (Left Half) (H&E 100*); B, Sheet of Highly Pleomorphic and Atypical Cells without Differentiation (H&E 400*); C, Vascular Proliferation and Tumoral Necrosis (H&E 100*); D, Palisading Necrosis (H&E 400*).
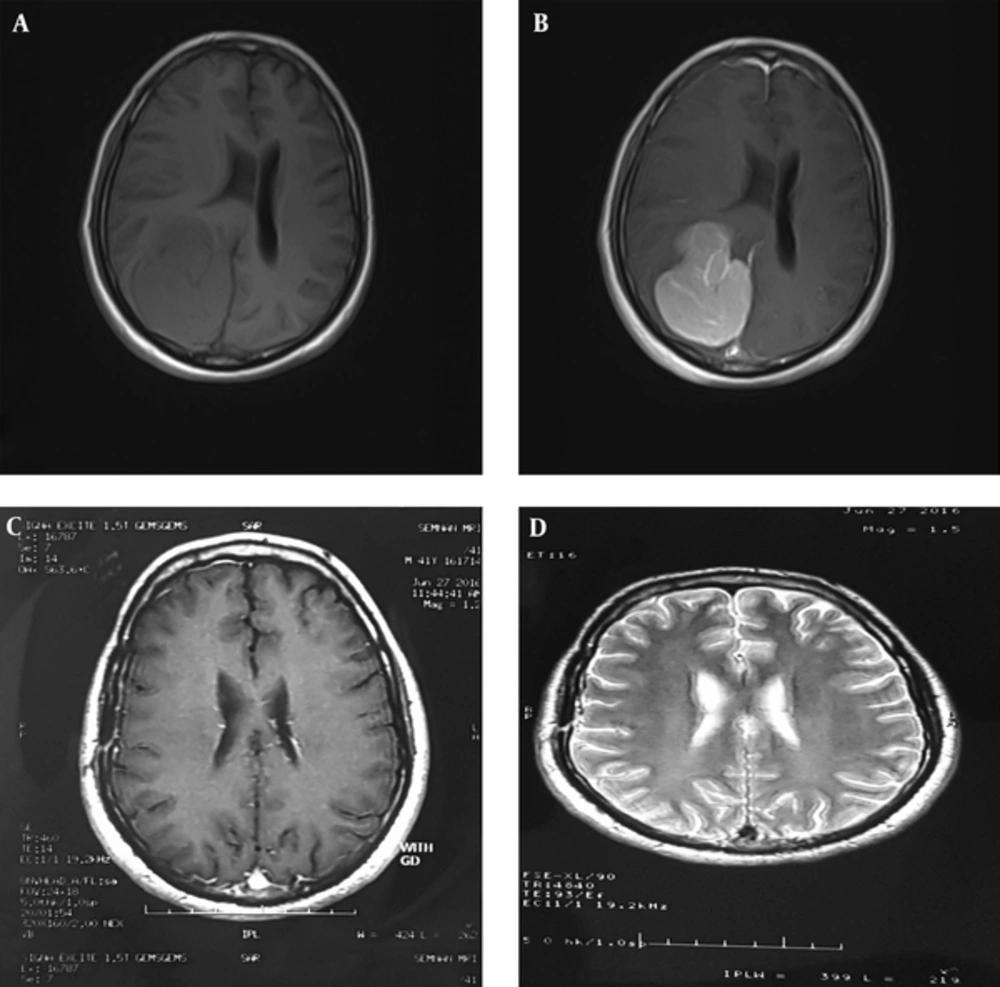
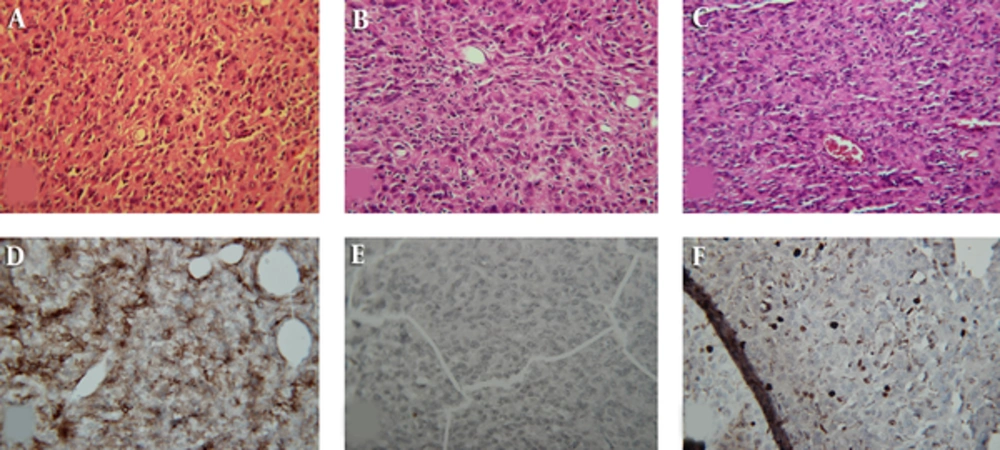
The second patient had a reverse fate. He was a 52-year-old male manifesting with headache and pain in the right ear. The physical examination was normal. MRI revealed a hyper-signaling homogenous mass on right parietal lobe with peripheral edema and contrast enhancement (Figure 3). These imaging findings were consistent with meningioma; however, the pathologist reported an anaplastic astrocytoma as an initial diagnosis. Because of imaging/pathologic discordance, pathological review was requested. The review showed a moderate hypercellularity pattern without any macronucleoli and necrosis (Figure 4). The immunohistochemistry showed a positive epithelial membrane antigen-a (EMA) and negative cytokeratin (CK) plus a ki67 of 710% (Figure 4). If the patient had been managed based on the primary diagnosis of anaplastic astrocytoma, he should have been treated by 60 Gy and most probably adjuvant chemotherapy. However, he escaped from this heavy treatment by a definitive diagnosis of meningioma.
Pre- and Post-Operative MRI of the 2nd Patient; A (Pre-Op), T1 Study of Brain Presenting a Well Circumscribed Hypointense Mass in the Right Parieto-Occipital Region with Midline Shift; B (Pre-Op), Contracted T1-Imaging Study of Brain Showed Homogenous Contract Enhancement with a Nonspecific Meningeal Tail; C and D (Post-Op), T1- and T2-Imaging of Patient, 4 Months After Surgery, Revealing Complete Resection.
4. Discussion
The current descriptive study aimed at investigating the discordance between imaging data and pathologic diagnosis of patients with brain tumors. Imaging/pathologic discordances were found in 11 out of 240 patients, which in 82% of them was associated with a change in diagnosis. The change in the final diagnosis led to considerable alterations in the management of patients with brain tumors. For instance, the final diagnosis of patients with an initial diagnosis of medulloblastoma changed to GB or diagnosis of patients with initial diagnosis of meningioma changed to anaplastic astrocytoma.
In practice, initial pathologic diagnosis in patients with brain tumor is mostly not the definitive final diagnosis from the pathologist’s point of view; brain neoplasms appear heterogeneous with a considerable within tumor and among tumor histopathological variation (15). Necrosis, vascular proliferation, and increased mitotic activity are considered as features of high grade tumors; however, it is not always straightforward. Several events lead to misdiagnosis including errors during biopsy, incorrect interpretation of microscopic features of tumoral tissue, and the interobserver variation during pathologic assessment (5, 10). A study by Pant et al. in 2015 showed that discordance between radiologic findings and pathologic reports was found in about 3% of brain tumors (16). In the current study, glioblastoma was the disease with the highest imaging/pathologic discordance.
Glioblastomas typically present with central necrosis, extensive peripheral edema, and heterogeneous contrast enhancement in imaging studies; however, in the current study the initial pathologic report of the mentioned patients was the low-grade tumors such as astrocytoma. Also, imaging/pathologic discordance frequently happened in differentiation of various subtypes of neuroepithelial brain tumors, especially gliomas. Pant et al. showed similar results with no relationship between pathologic definitive diagnoses and imaging findings (16). In a study by Iwama et al. in the 1990s, there was no correlation between signal intensities of gliomas in magnetic resonance imaging and grade of tumors (17). It seems that interobserver variation during pathologic assessment of glioma is very critical (8-10).
For more accurate diagnosis, it is recommended that pathologists prepare slides from the entire surfaces of the tumoral tissue for microscopic evaluation of brain tumors. Whole paraffin block assessment approach may increase the accuracy of pathologic diagnosis of brain tumors by improving the histopathologic features appraisal of tumor; however, this might be accompanied by inadequate proof for definitive diagnosis. In this case, pathologists usually report an inconclusive or suggestive diagnosis (5). Consequently, under such circumstances, clinicians should judge based on microscopic description of tumoral tissue (instead of relying on the absolute final diagnosis reported by pathologists), clinical presentation of patient, and imaging findings. Thereafter, if there is discordance, further pathologic consultations with an expert neuropathologist besides using relevant immunohistochemistry protocols should be requested (18). It is believed that another key point to decrease the discordance between pathologic diagnosis and clinical findings is maximal tumor safe resection. It leads enough tumor sample availability and consequently facilitates correct pathological confirmation.
Neuro-oncologists and neurosurgeons should have enough information on imaging findings interpretation and microscopic description of tumoral tissue to reconcile the findings. In addition, pathologists should also be familiar with radiologic features of brain tumor and consider them during reporting that may lead to a radiology-based provisional diagnosis. For this purpose, developing a diagnostic algorithm (at least based on the most prevalent tumors) could theoretically provide timely clinical decision support and prevent unnecessary testing (19). Using these diagnostic algorithms is strongly recommended, especially for the infratentorial intra-axial parenchymal and infratentorial extra-axial tumors (16).
Also, it is noteworthy that meningiomas are usually extra-axial and gliomas are intra-axial. All meningiomas are not the low-grade tumors, but some of them (including 6 histopathological subtypes) are reported as grade II and III.
4.1. Conclusions
The current study showed that imaging/pathologic discordances were detectable in 4.5% of admitted patients with intracranial neoplasms. The discordance led to a change in the final diagnosis in 82% of the patients. The practical importance of these findings was that the treatment approach and prognosis of the patients with brain tumors substantially varied across the different subtypes and grades. Considering the limited sources in the developing countries such as Iran, reviewing the pathology of all patients is not practical. Therefore, it is suggested that 3 main specialties involved in treatment of brain tumors (ie, neuro-oncology, neurosurgery, and pathology) should be familiar with all the related pitfalls.