1. Context
Nowadays, cancer is one of the serious public health problems around the world. It is now clear that elevated lactate levels are one of the common symptoms of many cancers. This review article utilizes 18 articles from the PubMed and Google Scholar databases. Research in the field of cancer display How this compound can affect cancer patients, and perhaps by controlling its high production, it relieves a great deal in treating cancer.
Lactate, a 3-carbon hydroxyl carboxylic acid, is produced by glycolysis in the cytoplasm under anaerobic conditions from pyruvate and conversion of NADH to NAD+ (1). Up to 40% of circulating lactate can be generated by skeletal muscle, which the liver and kidney generally receive from the bloodstream and turn into glucose (2). This terminal compound of pyruvate metabolism is carried out by the lactate dehydrogenase (LDH) enzyme (3). In this review, we will present the major findings of the articles about one of the most metabolites in cancer patients.
2. Evidence Acquisition
It is commonly acknowledged that lactic acidosis is a pathological condition, with a serum lactate concentration or lactic acid remaining above 5 mmol/L, while its normal concentration is 1 to 2 mmol/L (4). Blood lactate levels reflect the balance between its production and clearance. However, lactate is produced about 1500 mmol daily under normal physiological conditions in skeletal muscle (25%), skin (25%), brain (20%), intestine (10%), and red blood cells (20%) (5) and also in patients from other tissues such as lung, leukocyte, and visceral organs (6). Furthermore, its clearance is carried out in the blood mainly by the liver (60%) and the kidneys (30%), and the heart (10%) (7). Hyperlactemia (increased lactate) can occur under hypoxic or anemia conditions, and hypolactemia (reduced lactate) due to liver or kidney and heritable disorders or due to drug toxicity (8).
The following steps occur in its oxidation: (1) Lactate is passed into the cell by the monocarboxylate transporters (MCTs) or produced within the cell; (2) LDH enzyme is required to convert lactate to pyruvate in both directions then the pyruvate enters the tricarboxylic acid (TCA) cycle (9).
It is estimated that lactate provides up to 40% of the oxidative substrate required by the heart at rest, which increases by up to 60% with activity (10). It acts as a buffer between glycolysis and oxidative metabolism that, in the process, is exchanged as fuel between cells and tissues at different glycolytic and oxidative levels (11).
It should be noted that the brain is an extremely complex organ that changes the amount of energy it needs throughout life and increases during childhood but changes throughout life due to oxidative phosphorylation (12). Although glucose is a significant substrate in normal brain tissue, it has recently been shown that this tissue has a high ability to absorb lactate after exercise (13). Monocarboxylate transporters transport lactate and pyruvate through the plasma membrane. As well as this transition was possible in both pathways (14) along with lactate transfer, which also transports protons to maintain acidity inside and outside the cell (15). Fourteen subgroups of MCT have been identified in various body tissues and reported in various cancers expressing and regulating MCT1, MCT2, MCT3, and MCT4. MCT1 and MCT4 are expressed in skeletal muscle and MCT1 in the heart (16), MCT1, MCT2, and MCT3 in the brain (17).
2.1. Cancer
It has been shown that cancer cell metabolism has changed compared to normal cells, which is known as the Warburg effect (18). In these aberrant cells, glycolytic levels were much higher than in normal tissues, and they performed anaerobic glycolysis even in large amounts of oxygen. The effect of Warburg has revealed that some cancers cause loss of mitochondrial activity (19), which reducing mitochondrial activity is a valuable advantage to maintaining cancer cell survival by inhibiting apoptosis (20). High glucose uptake is a hallmark of cancer cells providing the energy and precursor needed to biosynthesize other molecules and proliferate in cancer cells (21). The increased expression of glucose transporters and glycolytic enzymes results from oncogenic signaling and their complex transcriptional networks (22).
It is also very important to remember glutaminolysis is another major pathway for energy production in cancer cells that ultimately leads to increased lactate production and facilitates the synthesis of macromolecules in tumor cell proliferation (23). However, numerous studies have shown that cancer cells use glycolysis and mitochondrial oxidative metabolism to meet their metabolic needs (24).
2.2. Lactate and Cancer
Despite early promising data, the tumor microenvironment is a complex network of extracellular matrix molecules, soluble factors, adipocytes, and stromal cells composed of tumor endothelial cells, tumor-associated fibroblasts, and macrophages. In tumorigenesis, all of these components work together in an unusually acidic environment (19).
Among the microenvironment-soluble factors, a large amount of lactate is important for its effects on tumor and stromal cells (1). The pH of the extracellular medium decreases from 6 to 6.5, resulting in high lactate production by cancer cells (25). Pathology of the tumor microenvironment is under the influence of several key physiological factors: oxygen transport, vascular structure, nutrition, pH, and metabolite transport (26). Lactate has a critical role as the terminal compound of aerobic glycolysis in the rapid proliferation of tumor cells without resemblance to normal cells (27). Elevated lactate production is one of the dominant features of altered tumor metabolism; increased intracellular lactate must be released externally to maintain intracellular pH (28). It has been shown that this extracellular acidic environment is induced by lactate, facilitating tumor cell invasion, metastasis, and immunosuppression, increasing cell survival and resistance to apoptosis (29). On the other way, lactate is released at physiological levels at points of infection and inflammation by leukocyte activity (30). One study has shown that some cancer cells use lactate as a respiratory substrate and a lipogenic precursor in a culture medium (31). Lactate produced in hypoxic areas of the tumor can be used to generate energy by tumor cells in areas near a blood vessel (32).
It must be considered that this chemical material, as molecular signaling runs important roles in stimulating tumor inflammation and facilitating tumor angiogenesis (33). Increased lactate alters the tumor microenvironment and fuels cells of cancer cells, which are involved in acidosis, inflammation, angiogenesis, immunosuppression, and radiation resistance. Aerobic glycolysis and glutaminolysis are accepted as key markers of cancer, and both are involved in the metabolic acidosis of solid cancers that leads to the production and secretion of lactate. The extracellular pH of the tumor can be reduced to 6.5 - 6.5 instead of the pH of 7.4 in normal cells. This, in turn, is associated with weight loss, loss of appetite, and cachexia (34).
MCTs are found in the cell membranes of a variety of cells, such as tumor cells (35), erythrocytes (36), and neutrophils (37). MCT1 is expressed at low levels in most tissues, and MCT2 and MCT3 are restricted to some tissues. MCT2 is expressed predominantly in the liver, kidney, and neurons, and MCT3 in retinal epithelial pigments. High levels of MCT4 are found in white skeletal muscle fibers and, to a lesser extent, in the testes, lungs. Also, expresses MCT4 in some cells, such as chondrocytes, leukocytes, and astrocytes (38). Lactate is released by glycolytic cells, such as astrocytes, and can be transferred to other cells, such as neurons, for oxidative metabolism (39). Some tumors, such as glioma, breast, colorectal, gastric, cervical, and neuroblastoma, show increased expression of MCT1 and MCT4 (40). Of course, glioblastoma cells rely on MCT4 for proliferation and survival (41).
2.3. Lactate and Immune Cells
The previous finding demonstrated that high lactate levels have an undeniable role in stopping the entry of immune cells into the tumor, damage to the metabolism and function of cytotoxic T lymphocytes in the tumor microenvironment (42); it also prevents the release of T lymphocytes cytokines (43). It is estimated that tumor cells produce up to 40 times more lactate than normal cells (44). The tumor microenvironment contains neutrophils, macrophages, and dendritic cells that can produce lactate (45).
Neutrophils contain few mitochondria that rely on glycolysis for ATP production, while resting macrophages often metabolize glucose via glycolysis rather than oxidation but hexokinases and glucose 6 phosphate dehydrogenase are highly expressed in activated macrophages, which indicates that high pentose phosphate pathways are high in them (46). Macrophages M1 and M2 are different in terms of metabolism and immune function. M1 macrophages are the first line of defense against bacterial infections and obtain their required energy through glycolysis, and M2 macrophages use oxidative metabolism to affect tissue repair and wound healing (47). Macrophage phenotype changes from M1 to M2 during tumor progression (48).
Inactive dendritic cells use fatty acids and glucose for oxidative phosphorylation (49), but stimulation of TLR in these cells increases glucose uptake and lactate production (50), and dendritic cell activity stops when drugs inhibit glycolysis, and these cells rely on Warburg metabolism to survive (49). Stimulation of T lymphocytes results in a rapid increase in the expression of glucose transporters (GLUT1), glucose uptake, and glycolysis (51). In addition, by stimulating T lymphocytes, glutaminolysis increases and decreases fatty acid beta-oxidation (52).
Therefore, both glycolysis and glutaminolysis increase lactate production within the T lymphocytes. Several studies have shown that acidosis results in loss of function of T lymphocytes. The function of these cells can be resumed by buffering in physiological quantities (34).
B lymphocytes play an important role in antitumor immunity as antigen-presenting cells. In terms of metabolism, these cells are distinct from T-type lymphocytes that do not alter their metabolism from oxidative phosphorylation to glycolysis by stimulation. Even though these lymphocytes use both pathways, inhibition of glycolysis or deletion of GLUT1 in B lymphocytes suppresses antibody production (52).
As well, lactate alters monocyte function and consequently suppresses the immune system in the tumor (53). Studies have recently supported that NK cell activity is inhibited by lactate produced from the tumor or low extracellular pH (54).
3. Results
Tumor cells can secrete anti-inflammatory cytokines and suppress the immune cell population in the tumor microenvironment, inhibiting immune responses (55). On the other hand, the microenvironment of the tumor environment can act as a trigger for pain in cancer patients (56); it also contributes to the metastasis of some cancers (57). Lactic acid stimulates metalloproteinases-9 (MMP-9) in murine B16 melanoma (58), VEGF-A in gliomas and glioblastoma cells (59), IL-8 expression in pancreatic adenocarcinomas (60), and ovarian carcinoma cells (61). Lactate per second activates angiogenesis through the VEGF/VEGFR2 signaling pathway and stimulation of endothelial cells through MCT-1, which initiates phosphorylation/degradation of IKβ, stimulation of NF-Kβ/IL-8 pathway, which induces cell migration and new vascular formation (62). Lactate indirectly facilitates the survival of hypoxic tumor cells in newly formed areas away from blood vessels (63), and it is highly correlated with cancer malignancy and will lead to poor survival in these patients (64). Exosomes are 30- to 100-nm microscopic cells of endocytotic origin containing microRNAs, proteins, metabolic enzymes, and structural proteins. Exosomes play important roles in cancer metastasis and carcinogenesis (65). Lactate may play a key role in releasing exosomes and being absorbed by other cells (66).
Another study suggests that lactate is a marker of poor blood flow and a measure of severe disease, and in an emergency, the predicted mortality may be hyperlactemia (serum lactate greater than 4 mmol/L), metabolic acidosis, along with symptoms of tissue anemia (67). In another study, lactate is a predictor of brain tumor grade, and elevated serum lactate levels may be useful as a non-invasive biomarker, which correlates with tumor grade or range of invasion and also can determine tumor progression or response to treatment (68). Research has characterized high lactate concentration as an undesirable clinical condition (69), and lactic acidosis contributes to the death of patients with some metastatic cancers (70).
Common standard treatments such as radiotherapy or some chemotherapy drugs are the induction of reactive oxygen species (ROS), which cause DNA/RNA damage, but lactate may cause cancer cells to resist these treatments. Targeting MCT will induce apoptosis of tumor cells due to intracellular acidosis or lactate accumulation and inhibition of its uptake by aerobic tumor cells. Therefore, angiogenesis, invasion, and tumor metastasis will be reduced (71). It is well known that inhibition of MCT4 can induce lactic acid accumulation within the cell, and consequently, cell death occurs in hypoxic tumor cells (72). The feasible role of lactate can be used as a biomarker to predict survival in cancer patients (73), and a study reported that lactate may be a quantitative biomarker in response to acute radiation therapy (74).
4. Conclusions
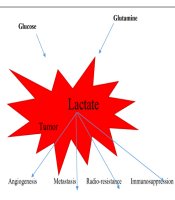
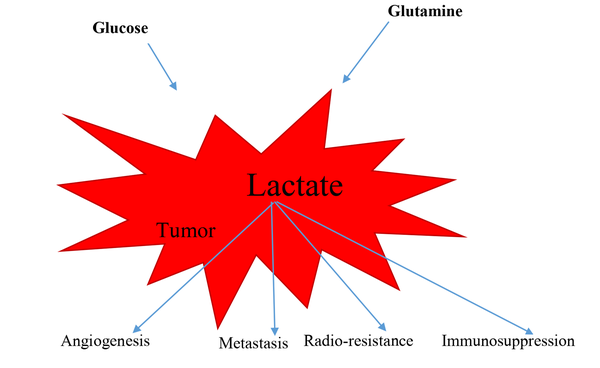
To put it in a nutshell, lactate, the most important metabolite, lead to angiogenesis in tumors, metastasis, resistance to radiation therapy and chemotherapy, and immunosuppression (Figure 1). Encouragingly, the levels of this effective substance should be monitored carefully, and it may be possible to reduce the mortality of this disease by affecting treatment worldwide.