1. Background
Nasopharyngeal carcinoma is an uncommon head and neck cancer, which is commonly seen in young people ranging in age from 15 to 25 years old. However, those aged between 50 and 59 years are at risk for this cancer too. The incidence of this cancer is more commonly seen in East Asian countries. Laryngeal cancer, on the other hand, is the second most common cancer among the head and neck cancers, which is far more common in men than in women (1).
The primary treatment for patients with nasopharyngeal carcinoma is radiotherapy. According to studies on the treatment of patients with nasopharyngeal cancer, radiotherapy with a dose higher than 70 Gy helps control the tumor in patients at stage T1-2. Radiotherapy for this tumor includes 2D and 3D techniques. However, 2D treatment is usually more routine. Studies conducted have shown that in 3D treatment, the tumor has a better volumetric coverage by the prescribed dose, reducing damage to healthy organs exposed to radiation (2, 3).
In laryngeal cancer radiotherapy, preservation of the vocal cord is very important during stages T1-T2 of the disease. Doses of 66 and 70 Gy are respectively prescribed for patients at stages 1 and 2. The usual treatment for this cancer is the use of the conventional technique. But the intensity-modulated radiation therapy (IMRT) technique has recently shown greater advantages over other radiotherapy techniques for laryngeal cancer, especially for patients undergoing extensive subglottic invasion (1).
In radiotherapy for neck cancer, the organs at risk include the thyroid, nerve cord, visual chiasm, etc., of which the thyroid is among the most sensitive organs at risk. In radiotherapy for tumors in the head and neck area, patients usually develop hypothyroidism due to thyroid radiation exposure. Studies have shown that levels of T3, T4, and TSH hormones change significantly in these patients. Hypothyroidism occurs in 12 - 53% of patients undergoing radiotherapy for neck cancer. But typically, between 15% and 30% of patients develop hypothyroidism within 4 weeks to 10 years after treatment (4-7).
In general, in tumors in the glottic region, where the thyroid may fall in the therapeutic field, it is essential to choose an appropriate radiotherapy technique to reduce doses received by the thyroid. Comparing the therapeutic techniques, including IMRT, VMAT, and 3DCRT in radiotherapy for glottic tumors, it was found that the thyroid receives the highest dose in the 3DCRT technique, whereas in other techniques, the planning target volume (PTV) provides better volumetric coverage and the thyroid receives lower doses (8).
Most studies on the effects of doses absorbed by the thyroid in radiotherapy for neck tumors have been conducted with the aim of investigating hormonal changes, and few have dealt with investigating the incidence of secondary thyroid cancer.
2. Objectives
This study was done to estimate the risk of secondary thyroid cancer using the biological effects of ionizing radiation (BEIR) VII model in radiotherapy for laryngeal and nasopharyngeal cancer.
3. Methods
This study used the dose received by the thyroid in a study by Salmanian et al. (9). In this study, treatment was designed for 20 patients with laryngeal and nasopharyngeal cancer, and simulation was performed on a phantom. Eventually, the thyroid dose was measured using TLD (TLD-100 chips (LiF: Mg, Ti), manufactured in Harshaw). The treatment was designed using posterior and anterior mediastinum by employing Alphard software on a human-like phantom. This phantom with a length of 95 cm and a thickness of 22 cm was made with a combination of Wax and NaCl, whose atomic number and density are similar to those of human body tissues (porous wood was used for lung tissue). The thyroid dose was measured three times for each treatment field related to nasopharynx and larynx. The prescribed dose was 40 Gy in 20 sessions in this study (9). The mean thyroid absorbed dose in the treatment fields of nasopharyngeal and laryngeal cancer in this study is presented in table Table 1.
This study used a BEIR VII model to estimate the risk of thyroid cancer risk (10). This model is designed based on the survivors of Hiroshima and Nagasaki atomic bomb attacks for low doses and low levels of linear energy transfer (LET). In order to estimate the risk of cancer, this model introduces lifetime attributable risk (LAR), which means the probability of dying from cancer because of the radiation exposure of that population.
Where, ERR is the incidence of disease in the exposed population minus that in the unexposed population. D, e, and a represent the mean organ dose, the age, at which the patient has been exposed to radiation, and the age, at which we want to calculate the incidence of cancer, respectively. Table 2 indicates other parameters, like BS , γ,and η depending on the type of model (EAR or ERR).
| B_Male | B_Female | γ | η | |
|---|---|---|---|---|
| Thyroid | 0.53 | 1.05 | - 0.4 | None |
Parameter Values for ERR in the Biological Effects of Ionizing Radiation (BEIR) VII Model
According to the BEIR VII model, the incidence of thyroid cancer does not depend on the age, at which we want to calculate the incidence of cancer (the attained age). Thus, in practice, thyroid ERR is a function of the absorbed dose and the age, at which the patient is exposed to radiation. In this study, the risk of thyroid cancer was calculated for patients aged 20 to 70 years undergoing radiotherapy for laryngeal and nasopharyngeal cancers.
4. Results
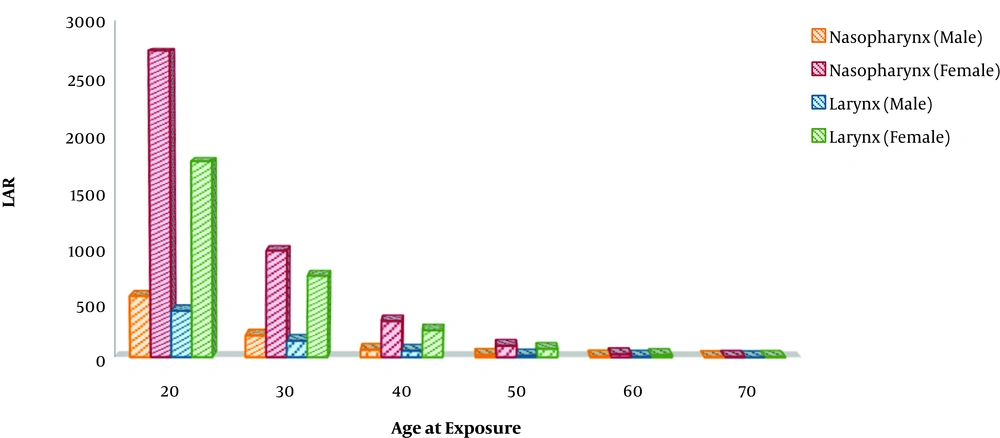
In both the nasopharyngeal and laryngeal treatment fields, the thyroid absorbed significant doses. The absorbed dose was 30% higher in the nasopharyngeal cancer treatment field than in the laryngeal cancer treatment field. LAR values in Figure 1 were obtained for a population of 100,000 people.
According to these results, the risk of thyroid cancer in men was about a quarter of that in women. The risk of thyroid cancer is much higher in patients undergone radiotherapy for cancers in the third and/or fourth decades of their lives than in patients in other age groups. The highest and lowest LAR values for the thyroid belong to women with nasopharyngeal cancer and men with laryngeal cancer, respectively. High values of thyroid LAR in nasopharyngeal radiotherapy can be due to higher exposure of the thyroid to the radiation field.
5. Discussion
This study dealt with investigating the risk of thyroid cancer in radiotherapy for thyroid cancer in the treatment of nasopharyngeal and laryngeal cancer. According to the results obtained, thyroid LAR increased up to 2.7% in the treatment of nasopharyngeal cancer and up to 1.8% in the treatment of laryngeal cancer. The highest LAR values belonged to the treatment of nasopharyngeal cancer. Since the prescribed dose and the number of treatment sessions were the same for both radiation fields, this increase in the dose received by the thyroid and consequently the increased risk of thyroid cancer could be due to higher exposure of the thyroid to the treatment.
In an investigation on the risk of secondary cancer in radiotherapy for breast cancer using a BEIR VII model in different treatment techniques, the results showed that radiotherapy increased the risk of thyroid cancer in patients undergoing breast cancer radiotherapy at the age of 40 years, whose thyroid received a dose within the range of 0.01 - 0.16 Gy in different treatment techniques, and the LAR increased up to 0.02% for these patients (11).
In another study conducted with the aim of assessing the risk of thyroid cancer in radiotherapy for neck cancers using the 3DCRT and IMRT techniques, the results showed that the thyroid received doses of 1.23 and 1.52 Gy, respectively. In the mentioned study, for patients who underwent radiotherapy at the age of 30 years, thyroid LAR levels increased up to about 0.3% in both techniques (12). Different LAR values in this study and our study can be due to differences in the thyroid absorbed dose and the S (a)/ S (e) ratio in the LAR formula of the BEIR VII model.
In a study on the risk of damage to the healthy tissue in radiotherapy for nasopharyngeal cancer in the IMRT and 3DCRT techniques using the NTCP model, it was found that the risk of thyroid damage was respectively 0.17% and 4.58% in the IMRT and 3DCRT techniques. The prescribed dose was 70 Gy in that study. The thyroid received a dose greater than 20 Gy in the IMRT technique, whereas it received a dose of about 40 Gy in the 3DCRT. The results of this study showed that the risk of damage to normal tissue is far less in the IMRT technique than in the 3DCRT technique (13). Comparing the 2DCRT, 3DCRT, and IMRT treatment techniques in the treatment of nasopharyngeal carcinoma, the results showed that IMRT was a more effective treatment technique in the treatment of this cancer at all stages than other therapeutic techniques (14).
Due to being exposed to the radiation field in radiotherapy for nasopharyngeal cancer, the thyroid is exposed to radiation damage. This damage can lead to changes in the hormones TSH and T4 and a decrease in the thyroid's volume depending on the dose received by the thyroid a few months after radiotherapy (15). Follow-up of patients with nasopharyngeal cancer treated with radiotherapy showed that 19% of them developed hypothyroidism 10 years after radiotherapy. The highest risk belonged to young female patients treated with the 3DCRT technique in comparison with those treated with the altered fractionation radiotherapy (AFRT) technique (16).
Considering the high radiation sensitivity of the thyroid, the risk of thyroid cancer is affected at low doses even within the diagnostic radiology range. In CT scans of the brain and sinuses, the thyroid receives a low dose; however, this low dose is effective in the risk of thyroid cancer. Thus, the effects of ionizing radiation on the incidence of thyroid cancer should not be ignored even at small doses (17). Therefore, it is essential to minimize the thyroid dose in radiotherapy for neck tumors using advanced treatment techniques. Moreover, in an investigation on damage to thyroid tissue in radiotherapy for head and neck tumors, it was found that the increased volume of the thyroid would reduce radiation damage to the thyroid in radiotherapy for head and neck cancers (18).
5.1. Conclusions
According to our study, radiotherapy for nasopharyngeal and laryngeal cancers considerably increased the risk of thyroid cancer in patients undergoing radiotherapy; such that the risk increased by more than 2.5% at younger ages. Due to the high radiation sensitivity of the thyroid and to prevent radiation damage in radiotherapy for neck tumors, it is recommended that an advanced treatment technique be used to reduce the dose received by the thyroid. Based on these results, it is recommended that patients undergoing neck cancers, such as nasopharyngeal and laryngeal cancer be followed up annually for the incidence of secondary thyroid malignancies and hormone disturbances.