1. Background
Colorectal cancer (CRC) is the third and fourth most common cancer in men and women worldwide, respectively. It is also the third most common cancer among women and the fifth most common cancer among men in Iran. Annually, more than 550,000 deaths in one million people are reported in patients with CRC (1). The prevalence and mortality rates of CRC are estimated to be high in developed countries and are also rapidly increasing in middle- and low-income populations (2). Globally, CRC accounts for 35% of all deaths due to cancer worldwide (3). According to the International Agency for Research on Cancer (IARC) report, the prevalence of CRC among men and women was 8.7 and 6.4 per 100,000 people in 2008, respectively (3).
The chemokine interferon-γ inducible protein 10 kDa (CXCL10) is a member of the CXC chemokine family, which exerts its biological effects by binding to CXCR3 receptor. It contributes to leukocyte trafficking (chemotaxis), apoptosis, cell growth, and angiostatic effects (4). It is also involved in several human pathological conditions, including infections, chronic and prolonged inflammation, immune dysregulation and dysfunction, tumor growth and development, and metastasis (4). Under proinflammatory conditions, CXCL10 is secreted from various cells, including leukocytes (e.g., activated neutrophils, eosinophils (5, 6), monocytes, endothelial and epithelial cells, and fibroblasts). This vital regulator of interferon (IFN) response chemoattracts activated Th1 lymphocytes to the inflammation site, followed by Th1 immune responses (5, 7, 8). CXCL10 is also involved in chemotaxis of monocytes, T cells, and natural killer cells. Due to the prominent role of CXCL10 in the recruitment of leukocytes to the inflammation site, it may deteriorate inflammation and tissue damage (9).
More importantly, CXCL10 has been identified as a major biological marker of disease severity and may be used as a prognostic marker for various diseases, particularly cancers. The CXC family of chemokines is unique, as it can induce or inhibit angiogenesis, depending on the presence of glutamine-leucine-arginine (ELR) or ELR sequence in the molecular structure. CXCL10, which lacks the ELR sequence, inhibits angiogenesis and exerts antitumor effects (10-12). Nevertheless, high expression levels of CXCR3 and CXCL10 receptors have been reported in some advanced human cancers, such as malignant melanoma (13), ovarian carcinoma (14), multiple myeloma (15), B cell lymphoma, and basal cell carcinoma (16).
Generally, biomarkers play an important role in the diagnosis, prognosis, treatment, and monitoring of cancers. The use of serum tumor markers can be a simple and noninvasive method for cancer diagnosis and assessment of prognosis and patient survival (17). Studies in recent years have found promising molecular biomarkers to predict the prognosis of CRC patients, although none of these studies can be confirmed, and further research is essential. Besides, most previous studies have been performed on the carcinoembryonic antigen (CEA), which is known as a predictive marker of prognosis and relapse in patients. Among cytokines, CXCL10 is also known to play an important immunomodulatory role. It is also a chemotactic immune factor; therefore, it can be used as a prognostic factor in CRC patients (4).
2. Objectives
Based on the results of previous studies and the speculated relationship between the expression of the aforementioned biomarkers and the prognosis of CRC, the present study aimed to evaluate the level of CXCL10 as a serum biomarker in CRC patients to predict their prognosis and survival.
3. Methods
3.1. Study Population
This retrospective cohort study was performed on 70 patients with a histopathological diagnosis of CRC, presenting to the oncology ward of Razavi Hospital in Mashhad, Iran, between July 2018 and September 2019. The patients were followed-up for up to four years. At the onset of the study, the patients’ demographic data, as well as information regarding the stage of cancer, chemotherapy regimen, and duration of therapy, was recorded. Besides, information on the patients’ cancer status, including the primary tumor location, tumor size, histopathological type of tumor, degree of tumor differentiation, TNM stage, vascular and lymph node metastasis or invasion, and CEA level, was collected. On the other hand, patients with a metastatic disease at the time of diagnosis, a history of colorectal malignancy or surgery, inflammatory bowel disease, collagen vascular disease, and renal or hepatic failure were excluded from the study. Finally, the patient’s cancer status was documented regarding survival, tumor metastasis, and recurrence over four years.
3.2. Serum CXCL10 Measurement
The patients’ whole blood samples (10 mL) were collected from the brachial vein, transferred to the laboratory of Razavi Hospital, and centrifuged at 1000 rpm for 10 minutes. The plasma fraction was isolated and stored at -70°C until further analysis. The serum CXCL10 concentration was measured by a Boster Bio ELISA kit (USA) in September 2019.
3.3. Ethical Considerations
The present study was performed on pre-collected samples of patients, admitted to Razavi Hospital. Informed consent was obtained from the participants before the study. The patients’ information was kept strictly confidential and was not disclosed to anyone by the researcher (ethics approval code: IR.MUMS.REC.1396.527).
3.4. Statistical Analysis
Statistical analysis was performed in SPSS version 19. Data are presented as mean ± standard deviation (SD) and median (interquartile range) for continuous variables with normal and non-normal distributions, respectively, and as number (percentage) for nominal variables. Moreover, Kolmogorov–Smirnov test was used to assess the normal distribution of variables. Independent sample t-test and Mann–Whitney U tests were also used, respectively, to compare normally and non-normally distributed variables between the two groups. Besides, for comparison of more than two groups regarding normally and non-normally distributed variables, one-way ANOVA and Kruskal-Wallis tests were used, respectively. Besides, Pearson’s correlation test (for data with a normal distribution) and Spearman’s correlation test (for data with a non-normal distribution) were performed to examine the extent of correlation between two quantitative variables. All the tests were performed at a significance level of 0.05.
4. Results
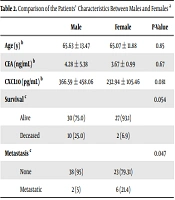
A total of 69 patients were included in this study. The mean age of the patients was 65.12 ± 12.75 years, and 41% of the study population were female. Twelve patients expired in this study after diagnosis during the follow-up. Four patients (6%) experienced recurrence, and 11% (9) had metastatic manifestations. The demographic data of the patients are summarized in Table 1.
According to the data presented in Table 2, the mean age of the patients was 65 ± 13.47 years in males and 11.88 ± 65 years in females (P = 0.85). The CEA and serum CXCL10 levels were not significantly different between males and females. However, the concentration of CXCL10 was lower in women than in men (P = 0.081). The results of this study showed that two female patients and 10 male patients expired during the study. Although there was no significant difference in terms of survival between men and women (P = 0.054), the incidence of mortality was higher in men than in women. The results revealed that six female patients and two male patients developed metastasis during the study. The results of statistical analysis also showed a significant difference between males and females regarding metastasis occurrence (P = 0.047).
The relative risk of mortality, metastasis, and recurrence in association with the serum concentration of CXCL10 is shown in Table 3. According to the Wald test, the serum CXCL10 level had a significant correlation with mortality and metastasis, but not with the recurrence rate. Similar results were obtained according to the Cox regression analysis. The normal range of CXCL10 in healthy individuals is between 24 pg/mL and 933 pg/mL. Accordingly, the CXCL10 concentrations were classified into two groups: A CXCL10 concentration > 933 pg/mL and a CXCL10 concentration < 933 pg/mL. The results of Cox regression analysis showed a significant relationship between the incidence of mortality and a high concentration of CXCL10 (P = 0.018). The results of Cox regression analysis also showed a significant relationship between metastasis and a high concentration of CXCL10 (P = 0.045). In other words, patients with a CXCL10 concentration above 933 pg/mL were more likely to develop metastasis. However, no significant relationship was found between the relative risk of recurrence and CXCL10 concentration in CRC patients during the four-year follow-up (P = 0.81) (Table 4).
| OR (95% CI) | Wald Test | P-Value a | |
|---|---|---|---|
| Mortality | 1.002 (1.0 - 1.003) | 5.119 | 0.024 |
| Metastasis | 1.002 (1.00 - 1.004) | 4.454 | 0.035 |
| Recurrence | 1.00 (0.997 - 1.004) | 0.05 | 0.82 |
Relative Risk of Mortality, Metastasis, and Recurrence in Association with the Serum CXCL10 Concentration in Patients with Colorectal Cancer During a Four-Year Follow-Up
| In a Normal Range (24 - 933) | Wald Test | P-Value a | |
|---|---|---|---|
| CXCL10 | Reference (< 933) | ||
| RR of mortality | 7.83 (1.412 - 43.127) | 5.548 | 0.018** |
| RR of metastasis | 17.12 (1.062 - 276.11) | 4.01 | 0.045** |
| RR of recurrence | 17.12 (1.062 - 276.11) | 0.056 | 0.81 |
Relative Risk of Mortality, Metastasis, and Recurrence in Patients with Colorectal Cancer During a Four-Year Follow-Up
5. Discussion
The results of the present study showed that the serum concentration of CXCL10 was significantly associated with the incidence of mortality and metastasis in CRC patients. Patients with a serum CXCL10 concentration above 933 pg/mL were more exposed to cancer-related mortality and metastasis. The results of Cox regression analysis showed no significant relationship between the relative risk of recurrence and CXCL10 concentration in CRC patients during the four-year follow-up.
Several studies have investigated the role of CXCL10 as a prognostic factor in CRC. In this regard, a study by Dimberg et al. in 2014 found that CXCL10 levels were significantly higher in CRC-affected tissues compared to the healthy tissues of CRC patients. Besides, the plasma level of CXCL10 was significantly higher in patients with CRC compared to the control group (18). It was concluded that assessment of changes in the plasma and tissue concentrations of CXCL10 in CRC patients may be useful in predicting clinical findings, such as recurrence, metastasis, lymph node involvement, and further pathological stages (18).
In a study by Jiang et al. in 2010, examining 64 stage II and III CRC patients, no significant correlation was found between the level of CXCL10 according to immunostaining and the tumor location, tumor size, histological type, lymphovascular invasion, perineural invasion, or TNM stage (19). However, patients with lower levels of CXCL10 expression showed a poorer prognosis (19). In another study by Zipin-Roitman et al. on CRC cell lines, the expression of CXCL10 and CXCR3 was induced by IFN-gamma in these cells. However, this chemokine did not affect tumor growth characteristics or angiogenesis-related functions. CXCL10 also significantly increased the invasion-related properties of these cells (20).
Additionally, a study by Toiyama et al. in 2012 on 218 CRC patients and 17 healthy individuals showed that the serum CXCL10 levels increased as the disease progressed in CRC patients (21). The serum CXCL10 level was also significantly associated with the pathological stage of tumor, vascular invasion, and distant metastasis. Also, high levels of CXCL10 had a significant association with low survival in all stages of CRC, which is consistent with the findings of the present study (21). A study by Bai et al. in 2016 on 143 patients with stage II CRC found that the overexpression of CXCL10 and CXCR3 could be prognostic factors in patients and might be associated with clinical outcomes, such as recurrence and lymph node involvement (22).
Moreover, a study in 2012 by Wu et al. on patients with CRC found that CXCR3 mRNA expression was significantly higher in the primary colorectal tumor tissue compared to non-cancerous colon tissue (23). The expression of CXCR3 was detected in 98 out of 112 patients. Therefore, CXCR3 may play a key role in the development of CRC. By analyzing the correlation between the clinicopathological factors and the expression of CXCR3, it was concluded that a high level of CXCR3 expression was significantly correlated with tumor differentiation, tumor size, lymph node involvement, and distant metastasis. Also, patients with high levels of CXCR3 expression had lower survival rates than patients with lower CXCR3 expression levels. The results of this study are in line with the findings of a recent study, which showed a significant relationship between the serum CXXL10 level and mortality and metastasis (23). Another study in 2014 by Comstock et al. on 126 asymptomatic men revealed that high levels of CXCL10 significantly induced tubular adenomas in patients (24). It was also found that in patients with more than three colorectal polyps, CXCL10 showed an increasing pattern (24).
As mentioned earlier, available studies on the serum level of CXCL10, as a prognostic factor in CRC patients, have reported some controversial findings, and further detailed research is essential. One reason for the contradictory results of these studies is the presence of some important confounding factors, such as metastatic disease at the time of diagnosis, history of colorectal malignancy or surgery, inflammatory bowel disease, collagen vascular disease, and renal or hepatic failure. However, in the current study, these cases were considered as the exclusion criteria. Unlike other studies, we attempted to exclude all important confounding factors influencing the serum concentration of CXCL10. Therefore, the main difference between the present study and previous research is the higher accuracy of this study in the evaluation of CXCL10 as a prognostic factor by removing the confounding factors.
Another possible mechanism to explain the difference between the results of these studies is the dual role of CXCL10 in cell growth. The proliferative and anti-proliferative effects of CXCL10 are cell-specific and related to the receptor subtype expressed at the cell surface. There are three variants of CXCR3, including CXCR3-A, CXCR3-B, and CXCR3-alt. Different cells express different patterns of these three receptor isoforms. Besides, different CXCR3 isoforms mediate the opposing effects of CXCL10 on proliferation. The anti-proliferative effects of CXCL10 are mediated by the CXCR3-B isoform. This receptor couples with Gαs, activates adenylate cyclase, and inhibits endothelial cell proliferation and migration (25); this seems to be the key mechanism in the anti-proliferative effects of CXCL10. Moreover, this receptor subtype does not result in chemotaxis (25). Cells that contain this receptor include endometrial malignancies, glioblastoma, and colorectal malignancies. The migration of CXCR3-positive T cells to inflammatory or neoplastic regions is mediated by CXCL10 and is also associated with the inhibitory effects of tumor progression and anti-metastasis effects (26).
The CXCR3-B isoform, as a common receptor for all four types of angiostatic chemokines (CXCL4, CXCL9, CXCL10, and CXCL11), provides a better understanding of the role of CXC chemokines in inflammatory cells. It is known to regulate inflammatory responses, leading to angiostasis and inhibition of endothelial cell proliferation (27). Besides, CXCL10 lacks the ELR sequence, inhibits angiogenesis, and exerts antitumor effects (4). However, the high expression of CXCR3 and CXCL10 receptors has been observed in some advanced human cancers, such as malignant melanoma (13), ovarian carcinoma (14), multiple myeloma (15), B-cell lymphoma, and basal cell carcinoma (16). In the CNS microglia, astrocytes and even neurons express and secrete CXCL10. CXCL10 results in the chemotaxis of microglial cells into the CNS circulation. The CXCL10/CXCR3 signaling also increases in some CNS-related pathological conditions (28). Generally, studies on a larger sample size of patients, considering all characteristics of patients that may be involved in the prognosis of cancer, are recommended for reaching a more definitive conclusion.
5.1. Conclusions
The results of this study showed that the serum concentration of CXCL10 was significantly associated with the mortality and metastasis of CRC patients.