1. Background
Osteosarcoma as a malignant tumor originates from spindle-shaped bone or soft tissue cells producing malignant osteoid. The precise epidemiology of osteosarcoma in Iran remains unknown. Yet, studies have reported that osteosarcoma is implicated in 15.1 - 50.6% of the primary bone tumors in Iran (1, 2). It is the most common primary bone malignancy after hematopoietic malignancies and occurs mostly in bones that grow rapidly. It is mostly expected to occur in the second decade of life. Before the development of chemotherapy regimes in the early 1970s, the prognosis of this disease was very poor, with a five-year survival rate with surgery of only 20%. Currently, with extensive and modern adjuvant chemotherapy, the treatment results of this malignancy have changed favorably, so the 5-year survival rate without recurrence is between 60 and 70% (3). Usually, distal femur, proximal tibia and humerus are affected by this tumor. Because of its aggressive and rapidly progressing nature, the classic treatment was limb amputation. However, recently, the prognosis of osteosarcoma patients has miraculously altered. Effective chemotherapy drugs have significantly decreased the rate of metastasis and mortality. Many investigations have proven a direct link between the evolution of chemotherapy and increased life expectancy. In addition, recent progresses in imaging and prosthetics has created more freedom for the surgeons in determining the preoperative treatment method. These advances have created less invasive, more definitive, and better treatment methods. Surgeries, like hip disarticulation and radical amputation contradict the aforementioned tendencies and have become a subject for more important studies to investigate the psychological function and prognosis of these operations (4). Recently, with the emergence of neoadjuvant chemotherapy, limb salvage has become the basis of osteosarcoma surgeries. However, limb salvage techniques might increase the risk of insufficient resection of the surgical margin (5). Randomized and non-randomized studies have proven chemotherapy’s primary role in treating osteosarcoma without metastasis (6). The incidence of this disease is about 1 - 3 per million people annually and is observed at any age. Nonetheless, high-grade primary tumors commonly occur in the second decade of life. Parosteal osteosarcoma has the maximum rate of occurrence in the third and fourth decades of life. In comparison, secondary osteosarcoma occurs more in elderly. Men exhibit slightly higher incidence, except for parosteal osteosarcoma which affect women more commonly. No significant difference is observed between races, and genetic factors rarely are involved in the prevalence of this disease (7). The appearance of osteosarcoma radiographs is varied, but plain radiographs have been the most reliable tool for accurate diagnosis. An aggressive lesion in the metaphysis of long bones is commonest radiographic appearance of the disease. Nearly 10% originate from the diaphysis and less than 1% from the epiphysis (8). The lesion can be predominantly lytic or blastic but, regions of bone formation as well as destruction are both commonly seen in these lesions. This lesion is completely permeative, and the edges are not very clear. If the lesion has destroyed the cortex, at the time of diagnosis, a soft tissue mass can be observed. MRI can define the extent of soft tissue invasion and could be used in limb salvage preplanning.
In this study we share our 10-year experience in the management of osteosarcoma as the referral center for orthopedics in south of Iran.
2. Methods
In this retrospective cohort study, the files of all patients diagnosed with osteosarcoma in Namazi and Shahid Chamran hospitals from 2008 to 2017 were studied. Due to the lack of phone numbers or exact addresses of a large number of patients, out of the 182 records, only 56 patients were included. Inclusion criteria in this study was osteosarcoma patients who were confirmed by pathology and undergone surgical intervention. Exclusion criteria was unavailability of the patients’ medical record or poor follow up patients. It should be noted that out of this number, a significant number had regular clinic follow-ups. However, if the duration of their visits to the clinic was more than six months, these patients were referred to the Chamran hospital clinic for follow-up and included in this study. In the clinic, after taking a history of the disease and physical examination, they were asked to take a photo of the involved position and a chest X-ray. It is worth noting that taking these photos did not cost the patient. Then, the photos were examined, and in case of suspicion of local or distant metastasis, if the patient had a prosthesis and MRI sensitivity was low, a bone scan and CT scan were requested; otherwise, to confirm the presence of metastasis in the lung, CT scan, and the involved position, MRI was requested, and the professor carefully reviewed these graphs.
Of the 56 patients who were studied, 21 had died, and by asking the exact time of death and studying the patient’s file to find out the exact time of osteosarcoma diagnosis and treatment, the required information such as type of surgery. Time of diagnosis, location of tumor and survival rate after surgery and chemotherapy were recorded. Thirty-five patients were alive, and the necessary information on the treatment type as well as the course of the disease was obtained from the patient and his/her record. In cases the patient was a child who was unable to cooperate, this information was received from the parents. It should be noted that the reason for conducting this follow-up was fully explained to the patient or his/her parents, and in case of non-satisfaction, they were excluded from the study. The study was in accordance with the Declaration of Helsinki and approved by Shiraz University of Medical Sciences Ethics Committee (IR.SUMS.REC.1388.580). Written informed consents were provided by all the participants. All the evaluations were non-invasive and done free of charge, and the data of the patients were kept confidential. The obtained data were analyzed by independent t test and chi- squared test.
Continuous variables were expressed as mean ± standard deviation (SD). Categorical variables were reported as frequency and percentage. The normality condition of the quantitative variables was investigated by using the Kolmogorov-Smirnov test. Chi square test and Fisher’s exact test were used to access the relation between quantities’ variables. Independent t-test or Mann Whitney U test was used to compare differences of quantitative variables between binary qualitative variables, as appropriate. Kaplan-Meier method was used for survival analysis. The Kaplan-Meier estimator is a nonparametric estimator for estimating the survival function from time to event data. In medical research, it is often used to measure the fraction of patients alive for a certain period of time after treatment. The Kaplan-Meier curve is utilized to approximate the survival function from data that may have missing values, are censored, or truncated. Log rank test was applied for comparison of survival time according to sex. Statistical analysis was conducted using the SPSS version 11.5. P-value of less than 0.05 was considered as significant.
3. Results
In this study, 56 patients with osteosarcoma who visited Chamran and Namazi hospitals between 2008 - 2017 were studied. Among these patients, 23 (41.1%) were female and 33 (58.9%) were male. Descriptive information about the age of the patients and the time of diagnosis is given in Table 1.
| Variable | Min - Max | Mean ± SD | Median (Q1 - Q3) | Statistic | |
|---|---|---|---|---|---|
| (P-Value) | T | ||||
| Age at the time of study (y) | 0.858 | 0.18 | |||
| Males (n = 33) | 10 - 62 | 26.06 ± 13.44 | 23.00 (17.00 - 28.00) | ||
| Females (n = 23) | 12 - 68 | 26.74 ± 14.31 | 23.00 (17.00 - 32.00) | ||
| Total (n = 56) | 10 - 68 | 26.34 ± 13.68 | 23.00 (17.00 - 28.00) | ||
| Age at the time of diagnosis (y) | 0.939 | 0.08 | |||
| Males (n = 33) | 8 - 60 | 2300 ± 13.06 | 20.00 (15.00 - 24.00) | ||
| Females (n = 23) | 9 - 67 | 23.30 ± 15.29 | 19.00 (14.00 - 27.00) | ||
| Total (n = 56) | 8 - 67 | 23.12 ± 13.88 | 19.50 (15.00 - 24.75) | ||
Descriptive Information Related to the Age and Time of Diagnosis in the Examined Patients in General and by Gender
The most commonly affected organ was the distal of the femur, and the most common procedure was amputation. Six of these patients had only received chemotherapy, the reason for which was that they did not go for surgery. Information about the involved organ performed on the patients is reported in Table 2.
| Variable | No. (%) |
|---|---|
| Affected organ | |
| Distal femur | 32 (57.1) |
| Proximal tibia | 11 (19.6 |
| Humerus | 5 (8.9) |
| Shaft tibia | 5 (8.9 |
| Fibula | 1 (1.8) |
| Radius | 2 (3.6) |
| Type of procedure | |
| Amputation | 25 (44.6) |
| Wide resection and allograft | 10 (17.9) |
| Only chemotherapy | 6 (10.7) |
| Wide resection and prosthesis | 15 (26.8) |
Frequency Distribution of Involved Organs and Type of Procedure in the Patients
According to Table 3, amputation is the most common procedure performed in both groups of patients (living and deceased). Among the patients, 21 people (37.5%) died, and 35 (62.5%) survived. The median (standard deviation) interval between diagnosis and death in patients who died was 3.14 (1.95) years. Among the 35 patients who did not die, only 4 (11.4%) had local metastasis, and 31 (88.6%) did not. In addition, among these patients, six people (17.1%) had distant metastasis, and 29 people (82.9%) did not have this complication. All six patients with distant metastasis had lung metastasis, and two had abdominal metastasis in addition to lung metastasis. In deceased patients, the least procedure performed is wide resection and allograft (9.5%). While in patients who survived, only chemotherapy had the lowest percentage (8.6%).
| Type of Procedure | Patient Status | |
|---|---|---|
| Alive | Deceased | |
| Amputation | 13 (37.10) | 12 (57.10) |
| Wide resection and allograft | 8 (22.90) | 2 (9.50) |
| Only Chemotherapy | 3 (8.60) | 3 (14.30) |
| Wide resection and prosthesis | 11 (31.40) | 4 (19.00) |
| Statistic (P-value) | 0.320 | |
| Fisher’s exact test | 3.545 | |
Frequency Distribution of Procedures in the Patients Based on Survival Status a
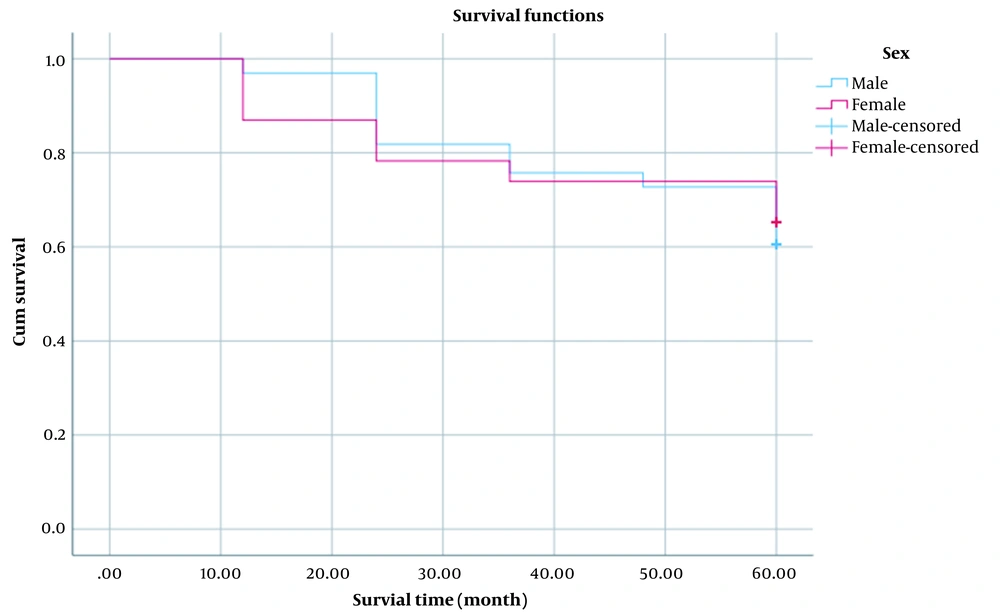
In order to compare the mortality ratio between males and females, the log-rank test was used, and no significant difference was found between the mortality ratio in females and males (P > 0.05). Also, the ratio of local and distant metastases showed no significant difference between males and females (P > 0.05) (Figure 1).
The mean 60-months survival time in men, women, and overall was 51.27 ± 2.74, 49.57 ± 4.05, and 50.57 ± 2.25. The log-rank test showed that the average survival time between men and women does not have a statistically significant difference (χ2 = 0.040; P-value = 0.842).
As shown in Table 4, the 1-, 3-, and 5-year survival rates of osteosarcoma patients are 93, 71, and 57%, respectively. According to Table 4, the 1-, 3- and 5-year survival rates of osteosarcoma patients in males are 97, 70, and 56%, respectively, which is not significantly different from the survival rate of all patients. As seen in Table 4, the 1-, 3-, and 5-year survival rates of osteosarcoma patients in females are 87, 74, and 58%, respectively, which is not significantly different from the overall survival rate of patients. Only, the difference in 1-year survival of males was significantly greater than that of females. Among the 56 patients, 15 (26.78%) survived for more than five years. Of the patients who died (21 patients), 18 (85.71%) had lower limb involvement, and 3 (14.28%) had upper limb involvement. The mean ± SD of survival years in patients with lower limb involvement was 3.28 ± 2.02 years and in those with upper limb involvement was 2.33 ± 1.52, but the difference between both groups was not significant. (P > 0.05). Of course, the non-significance of the difference between both groups could be due to the small sample size in the present study.
| Time | Male | Female | Overall |
|---|---|---|---|
| 1 year (12 month) | 0.97 ± 0.02 | 0.87 ± 0.06 | 0.93 ± 0.03 |
| 2 year (24 month) | 0.76 ± 0.06 | 0.78 ± 0.05 | 0.77 ± 0.06 |
| 3 year (36 month) | 0.70 ± 0.03 | 0.74 ± 0.04 | 0.71 ± 0.06 |
| 4 year (48 month) | 0.67 ± 0.02 | 0.74 ± 0.00 | 0.70 ± 0.06 |
| 5 year (60 month) | 0.56 ± 0.06 | 0.58 ± 0.08 | 0.57 ± 0.08 |
Estimated 1-, 3-, and 5-year Cumulative Survival in Osteosarcoma Patients
4. Discussion
Osteosarcoma is a primary and malignant bone tumor with the maximum incidence in the pre-pubescent age (3). The incidence of this tumor in the study conducted by Ghavam Nasiri et al., in a 15-year study, was not different from previous reports. The incidence of this disease is 1 to 3 per million per year (3). Although 27.8% of patients in the report of Ghavam Nasiri et al. had a history of trauma, such a relationship was not confirmed in other reports (3, 8). The osteosarcoma radiographic appearance can be very different, but plain radiography is the best diagnostic tool, and this result was also obtained in our research. The most common appearance of the tumor is an invasive lesion in the metaphysis of long bones (3, 8), and the lung is the commonest site of metastasis (9, 10), which we also found in this study. This research proved that the average patient age at diagnosis is slightly higher in females than in males, which was not statistically significant. Our study shows that gender does not affect patient mortality. The most common site of primary osteosarcoma occurs in places where the bone growth is higher, including the distal of the femur (10), and the result of our research also shows the same. In this study, the long-term survival of patients is 71%, and many reports have also reported long-term survival of 60 - 70% (11-13) and this good prognosis is due to neoadjuvant chemotherapy, surgical treatment, and then adjuvant chemotherapy. Such a protocol is also implemented in our center. In this study, 2-year survival is 77% for patients with no metastases at diagnosis; that is, only 22% of patients died during this 2-year period, while before chemotherapy, about 80% of patients died during this period (8). Due to the non-referral of patients or lack of accurate follow-up of chemotherapy treatment, the tumor has reached a stage that has involved blood vessels and nerves, and limb amputation has taken place. Eleven point four percent of patients had regional recurrence after surgery, which values of the same range (10%) have also been proven in other studies (14-16).
The surgical method of this group of patients includes extensive resection and continuation with chemotherapy (8, 15), for which we have also performed such a protocol for patients. In various studies, the chance of metastasis to the lung has been reported as 20%, and this possibility, that is, the possibility of metastasis to the lung, was 17.1% for patients treated in this center, which is a lower probability. In many studies, the most common operation performed for osteosarcoma is radical and wide amputation (16-18), and in this center, the most common surgery performed is amputation. In this research, it has been shown that age is not an influencing factor in metastasis and, consequently on, the rate of mortality.
4.1. Conclusions
There have been many advances in the treatment of osteosarcoma. A multifaceted approach to this disease, including surgery and chemotherapy has increased the possibility of better prognosis for this Adopting better treatment methods with more appropriate techniques, especially chemotherapy before surgery, timely surgery, continuous follow-up by the patient, and close cooperation of the treatment team consisting of orthopedists, oncologists, radiologists, social counselors, and psychologists to provide good treatment results should be recognized and used.
The limitation of our study was the fact that osteosarcoma is not a prevalent disease. This affected the number of our patients participating in our study. Furthermore, considering the costs of tumor prosthetics, a number of our patients undergone amputation despite being a candidate for salvage procedures. In future studies, researchers should come to a consensus regarding the precise treatment of osteosarcoma. Meticulous protocols must be adopted to help the surgeons choose between either salvage procedures of amputation.