1. Background
Breast cancer is now the most commonly diagnosed cancer globally, surpassing lung cancer, particularly among women (1, 2). Studies indicate that over the past two decades, the number of new cases diagnosed in both affluent and developing countries has been distributed fairly evenly (1, 3). Despite cancer traditionally being considered a disease in industrialized nations, approximately 45% of breast cancer cases and 55% of fatalities occur in nations with low to moderate incomes (4). Genetic and epigenetic alterations, including mutations of tumor suppressor genes, are among the factors contributing to breast cancer (5, 6). Moreover, the timing and stage of disease progression play a crucial role, with earlier diagnosis and intervention significantly impacting outcomes (7). Various intervention methods are available for different stages, ages, and histological grades of breast tumors (8, 9), with the stage determined by the extent of malignancy invading the breast tissue or spreading beyond the basement membrane (10).
In countries with limited resources, breast cancer is often diagnosed at an advanced stage despite medical advancements (11). Therefore, there is a strong emphasis on breast self-examination, as diagnostic ultrasound and mammography are limited resources (12).
Mammography screening is the most common method used worldwide to detect early breast cancer in asymptomatic women, and it has been shown to significantly reduce the mortality rate associated with breast cancer (13, 14). Socioeconomic and economic disparities are associated with a lower likelihood of cancer screening, late-stage diagnosis, and optimal treatment (15). The GLOBOCAN (the Global Cancer Observatory) database from 2014 revealed no significant differences between Iran and the global database regarding the incidence, mortality, or prevalence of breast cancer (16). Furthermore, genetic analysis has unveiled a wide array of molecular and genetic alterations in breast cancer, which may account for its diverse clinical behavior (17). Studies have identified molecular subclasses of breast cancer, such as luminal, basal, and HER-2.neu, as well as oncogene amplification. These subtypes exhibit significant variations in prognosis and response (18, 19).
This investigation was carried out to analyze the clinic pathological characteristics, treatment, and survival analysis of breast cancer patients who were referred to the Chemotherapy and Radiotherapy Department of Vasei Hospital, Sabzevar, Iran, during 2015 - 2019.
demonstrated that age, disease grade, lymphovascular/perineural invasion, the presence of necrosis, the kind of axillary lymph node surgery, and hormone therapy had no discernible influence on the overall survival of breast cancer patients. The overall survival of breast cancer patients was associated with disease stage, tumor molecular subtype, and chemotherapy regimen. disease-free survival in individual cases had a relationship with receiving modified radical mastectomy (MRM) and breast-conserving surgery (BCS).
The overall survival of breast cancer patients was associated with disease stage, tumor molecular subtype, and chemotherapy regimen. A further component of our study was that disease-free survival in individual cases had a relationship with receiving modified radical mastectomy (MRM) and breast-conserving surgery (BCS). Several studies have mentioned the role of increasing age as one of the most critical risk factors affecting the spread of breast cancer. Some studies conducted in Iran have reported the average age of cancer patients as 46.8, 48.4, 47.0, 46.8, and 49.0 years in descending order (20, 21). Numerous studies have underscored increasing age as one of the most critical risk factors influencing the progression of breast cancer. Additionally, it is evident that the incidence of breast cancer rises with age in both men and women, peaking in the age group of 70 to 74 years for women (22).
Moreover, considering that the current standard for breast surgery is breast-conserving surgery (BCS) and sentinel lymph node biopsy (SLNB), inadequate performance of axillary lymph node dissection (ALND) and inadequate assessment of the number of lymph nodes have impacted treatment decisions in many patients. Specifically, patients undergoing ALND had a maximum assessment of 10 lymph nodes, which may result in inaccurate staging and subsequently affect treatment decisions. Therefore, enhancing diagnostic and treatment facilities in healthcare centers and ensuring scientific updates in treatment services can lead to more effective treatment outcomes and improved survival rates.
2. Objectives
This study aimed to analyze the clinicopathological characteristics, treatment modalities, and survival outcomes of breast cancer patients referred to the Chemotherapy and Radiotherapy Department of Vasei Hospital, Sabzevar, Iran.
3. Methods
This retrospective longitudinal cohort study involved breast cancer patients referred to the Chemotherapy and Radiotherapy Department of Vasei Hospital in Iran from 2015 to 2019.
3.1. Inclusion and Exclusion Criteria
The inclusion criteria encompassed individuals aged 18 and above with a confirmed diagnosis of invasive breast cancer (excluding in situ cases like DCIS), whose medical records were present in the archives of the Chemotherapy and Radiotherapy Department of Vasei Hospital, one of the largest hospitals in the northeast of Iran. Patients referred during the specified period were eligible. Exclusion criteria included cases with incomplete information, those unreachable, and those unwilling to participate.
3.2. Study Design and Sampling
Data were extracted from the archives of the Chemotherapy and Radiotherapy Department of Vasei Hospital over a four-year period. A total of 275 medical records of breast cancer patients were evaluated. Clinical and pathological features of the tumors, prescribed treatments (chemotherapy, surgery, hormone therapy, and radiotherapy), overall survival, and disease-free survival information were reviewed. To adhere to ethical standards and maintain file confidentiality and patient privacy, only members of the research team conducted the file review, limited to relevant sections aligned with the research objectives.
Data extracted according to the prepared checklist included overall survival, disease-free survival, age group, menstrual status, history of diabetes, history of high blood pressure, personal and family history of breast cancer, family history of other malignancies, reason for referral, direction involved, tumor location, histology, grade, presence of in situ components, lymphovascular invasion, perineural invasion, presence of necrosis, TNM stage, molecular classification, type of primary tumor surgery, method of lymph node examination, chemotherapy approach, chemotherapy regimen, prescription of trastuzumab, and type of hormone therapy.
3.3. Data Analysis
Data were analyzed by SPSS software (version 22) using descriptive statistics, including frequency, percentage, mean, and standard deviation. In addition, Kaplan-Meier curves and log-rank tests were used for survival analysis. Regarding the inferential statistics, univariate and multivariate regression were utilized. A P-value of less than 0.05 was considered statistically significant.
3.4. Ethical Considerations
The study protocol was approved by the Ethics Committee of Sabzevar University of Medical Sciences (IR.MEDSAB.REC.1399.132). During information extraction, all data were coded to maintain confidentiality.
4. Results
This study analyzed 275 female patients with breast cancer. Due to in situ breast carcinoma (without an invasive component), three patients were excluded. Finally, a total of 272 female patients with invasive breast carcinoma were included in this study. The mean age of the patients was 49.35 years with a standard deviation of 11.02, and the majority of cases were in a premenopausal state (65.1%). Table 1 presents the demographic characteristics of the breast cancer patients. Breast cancer was predominantly detected on the right side (n = 141; 51.8%) and the outer upper quadrant of the breast (n = 140, 51.5%). Table 2 illustrates the pathological information of malignant masses.
| Variables | Values a |
|---|---|
| Age | |
| ≤ 35 | 21 (7.7) |
| 36 - 54 | 167 (61.4) |
| ≥ 55 | 84 (30.9) |
| Menstrual status | |
| Premenopause | 177 (65.1) |
| Perimenopause | 8 (2.9) |
| Postmenopause | 87 (32) |
| History of diabetes | 33 (12.1) |
| History of hypertension | 59 (21.7) |
| History of breast cancer | 2 (0.7) |
| History of family breast cancer | 10 (3.7) |
| History of other malignancy | 14 (15) |
| Reasons for referral | |
| Palpation of a lump in the breast | 268 (98.5) |
| Ulcer | 3 (1.1) |
| Nipple discharge | 1 (0.4) |
Demographic Characteristics of Patients
| Variables | Values a |
|---|---|
| Histology | |
| Ductal | 252 (92.6) |
| Lobular | 20 (7.4) |
| Grade | |
| 1 | 35 (12.9) |
| 2 | 151 (55.5) |
| 3 | 86 (31.6) |
| In situ component | 165 (60.7) |
| Lymphovascular invasion and perineural invasion | 168 (61.8) |
| Necrosis | 42 (15.4) |
Pathological Information of Malignant Masses
Additionally, most patients were in stage two (54.1%), with a median size of the primary breast tumor measuring 1.3 cm (ranging from 1 to 14 cm, with a mean of 3.7 ± 2.1 cm). The median number of lymph nodes removed during resection was 8 (ranging from 1 to 37, with a mean of 8.3 ± 5.8); however, in 23% of patients (n = 63), no lymph nodes were pathologically examined despite undergoing ALND. Conversely, in 38.2% of patients undergoing ALND, the maximum number of assessed nodes was 10 lymph nodes. In other words, the number of dissected lymph nodes in 61.8% of patients was insufficient for accurate axillary staging, rendering the reported N classification inadequate. Tumor staging and molecular classification are detailed in Table 3.
| Staging and Molecular classification | Values a |
|---|---|
| Staging | |
| Tumor, nodes, and metastases staging | |
| I | 15 (7.2) |
| II | 113 (54.1) |
| III | 69 (33) |
| IV | 12 (4.4) |
| T (tumor) | |
| I | 69 (25.4) |
| II | 158 (58.1) |
| III | 21 (7.7) |
| IV | 24 (8.8) |
| N (node) | |
| 0 | 60 (29.3) |
| I | 81 (39.5) |
| II | 49 (23.9) |
| III | 15 (7.3) |
| M1 (metastases) | 14 (5.1) |
| Molecular classification | |
| Molecular classification | |
| Luminal A | 95 (34.9) |
| Luminal B | 62 (22.8) |
| Her 2+ and hormone + | 52 (19.1) |
| Her 2+ and hormone - | 26 (9.6) |
| Negative triple | 37 (13.6) |
| Group molecular classification | |
| Luminal A/B | 157 (57.7) |
| HER2-enriched | 78 (28.7) |
| Triple-negative breast cancer | 37 (13.6) |
| Estrogen receptor status | |
| Positive | 67 (24.6) |
| Negative | 205 (75.4) |
| Progesterone receptor status | |
| Positive | 90 (33.1) |
| Negative | 182 (66.9) |
| Human epidermal growth factor receptor 2/neu status | |
| Negative | 63 (23.2) |
| Amplified | 209 (76.8) |
Patient Staging Information and Molecular Classification
Modified radical mastectomy (MRM) was performed in 55.4% of patients for breast lesion surgery, while breast conservation surgery was carried out in 44.6% of cases. Additionally, ALND was conducted in 233 (86.9%) patients, whereas SLNB was performed in 35 (13%) cases.
The chemotherapy approach administered to patients included adjuvant (88.2%), neoadjuvant (9.9%), and palliative (8.1%) treatments. In total, 229 (84.2%) patients received the doxorubicin hydrochloride and cyclophosphamide regimen (AC); additionally, trastuzumab was prescribed for 66 patients. Among the 208 patients who underwent hormone therapy, 75% were treated with tamoxifen, while 25% received aromatase inhibitors.
The overall mortality rate was 23.3% (n = 59), with local or distant recurrence observed in 28% of cases. The primary causes of death included coronavirus disease 2019 infection (n = 4) and active malignant disease (n = 55). Eight patients experienced disease recurrence, primarily in the form of leucorrhea from previous surgery or regional lymph nodes. Metastatic lesions, either present at the onset or during the disease course, were predominantly found in bone (n = 39), brain (n = 25), liver (n = 21), and lung (n = 19), in descending order.
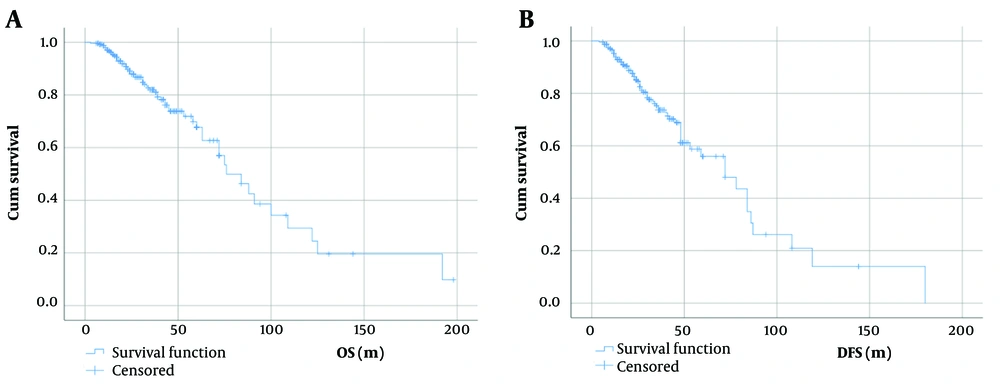
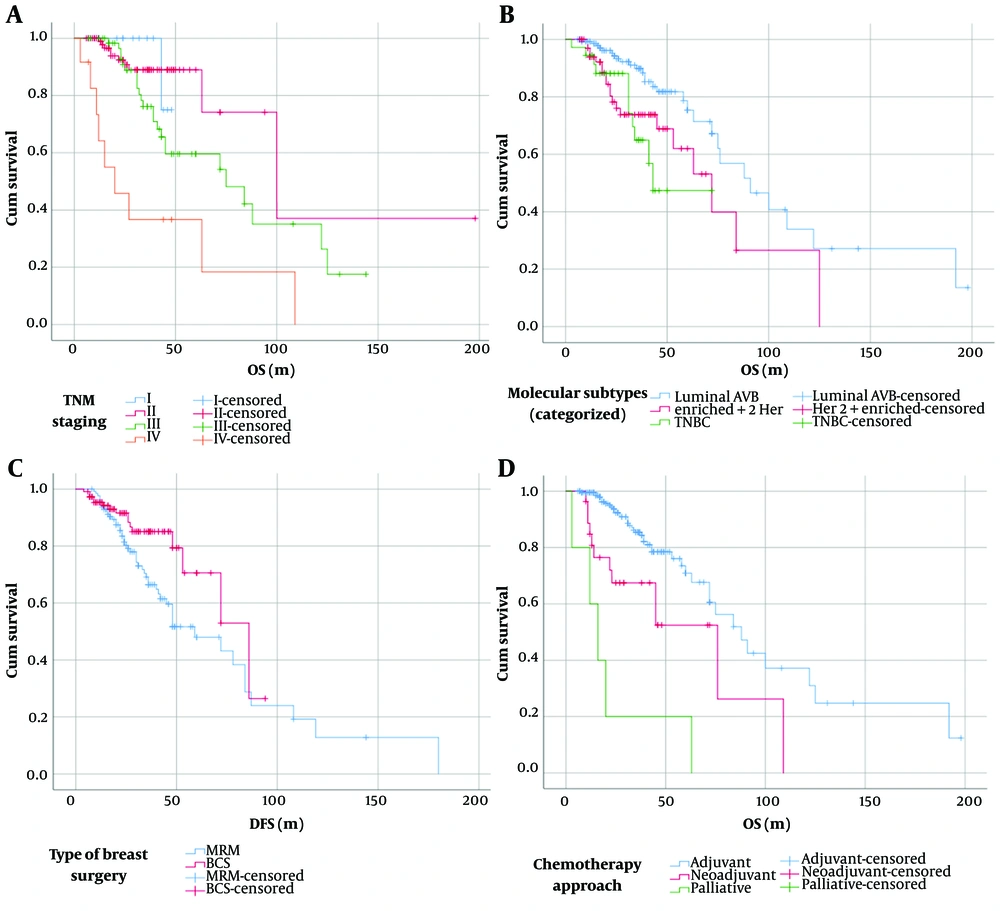
The overall survival rate of breast cancer patients, taking into account disease stage, tumor molecular subtypes, type of breast surgery, and chemotherapy approaches, is depicted in Figure 1 and detailed in Table 4. Overall survival did not significantly differ among patients of various age ranges (P = 0.87) (Figure 2).
| Variables | Mortality Rate | Mean Overall Survival, Month | P-Value |
|---|---|---|---|
| Age, (y) | 0.87 | ||
| < 25 | 20 | 53.9 ± 5.3 | |
| 26 - 54 | 158 | 93.7 ± 11.1 | |
| > 55 | 75 | 100.3 ± 15.2 | |
| Grade | 0.33 | ||
| 1 (n = 31) | 8 | 110.9 ± 24.8 | |
| 2 (n = 142) | 33 | 72.4 ± 6.3 | |
| 3 (n = 80) | 18 | 111.1 ± 14.2 | |
| Lymphovascular/perineural invasion | 0.52 | ||
| Yes (n = 152) | 39 | 84 b | |
| No (n = 101) | 20 | 75 b | |
| Necrosis | 0.3 | ||
| Yes (n = 40) | 11 | 10 b | |
| No (n = 213) | 48 | 76 b | |
| Stage of the disease | < 0.005 | ||
| I (n = 15) | 1 | 46.7 ± 1 | |
| II (n = 101) | 10 | 122 ± 28.5 | |
| III (n = 64) | 24 | 80.1 ± 8 | |
| III (n = 12) | 9 | 40.3 ± 12.9 | |
| Tumor molecular subtypes | 0.002 | ||
| A/B (n = 147) | 28 | 106.4 ± 11 | |
| HER2-enriched (n = 70) | 20 | 70.3 ± 9.2 | |
| Triple-negative (n = 36) | 11 | 50.1 ± 4.9 | |
| Type of breast surgery | 0.02 | ||
| Modified radical mastectomy (n = 138) | 43 | 75 | |
| breast-conserving surgery (n = 110) | 11 | 88 b | |
| Chemotherapy approaches | 0.0001 | ||
| Adjuvant (n = 240) | - | 88 | |
| Neoadjuvant (n = 27) | - | 76 | |
| Palliative (n = 5) | - | 16 |
Multiple Regression Analysis of Age, Grade, Type of Breast Surgery, and Type of Chemotherapy a
Table 5 displays the results of a single-variable test to predict disease recurrence. Multiple regression analysis revealed that the chemotherapy approach (β = 1.67, P = 0.02), T tumor type (β = 1.64, P = 0.01), N tumor type (β = 2.08, P = 0.005), Ki-67 (β = 0.02, P = 0.006), and RT field (β = -0.01, P = 0.003) were significant predictors of recurrence. Similarly, chemotherapy approach (β = 1.67, P = 0.02), T tumor type (β = 1.67, P = 0.01), N tumor type (β = 2.08, P = 0.005), Ki-67 (β = 0.02, P = 0.006), and RT field (β = -0.01, P = 0.003) significantly predicted mortality rates. Additionally, the chemotherapy regimen (β = -2.88, P = 0.01) and history of breast cancer (β = 73.21, P = 0.000) were identified as predictors of disease-free survival. Moreover, type of breast surgery and history of breast cancer were predictive of overall survival (β = -11.75, P = 0.02 and β = 96.17, P = 0.001, respectively).
| Criterion Variables | ||||||||
|---|---|---|---|---|---|---|---|---|
| Predictor Variables | Recurrence | Mortality | Disease-Free Survival | Overall Survival | ||||
| B | P-Value | B | P-Value | B | P-Value | B | P-Value | |
| Age group | 1.642 | 0.051 | 1.224 | 0.430 | 0.105 | 0.430 | 0.058 | 0.7 |
| Gender | 4219529259.000 | 0 .999 | 6.67 | 0.124 | -9.975 | 0.538 | -1.497 | 0.92 |
| Menopausal status | 1.467 | 0.21 | 1.161 | 0.639 | 3.442 | 0.283 | 1.194 | 0.74 |
| Laterality | 1.182 | 0.557 | 1.196 | 0.54 | 2.538 | 0.285 | -0.551 | 0.87 |
| Personal history of Breast Cancer | 0.000 | 0.99 | 0.000 | 0.99 | 76.231 | 0.000 | 72.412 | 0.000 |
| Family history of breast cancer | 0.271 | 0.220 | 0.712 | 0.66 | 1.050 | 0.887 | -4.995 | 0.55 |
| Family history of cancer | 0.000 | 0.999 | 0.999 | 0.00 | -10.931 | 0.105 | -12.627 | 0.1 |
| Diabetes | 0.867 | 0.744 | 0.858 | 0.73 | 6.349 | 0.147 | 4.52 | 0.37 |
| Hypertension | 0.554 | 0.126 | 0.606 | 0.21 | -2.233 | 0.535 | -5.973 | 0.15 |
| Histology of tumor | 1.061 | 0.914 | 1.845 | 0.21 | -0.739 | 0.898 | -0.647 | 0.91 |
| Invasive component | 648069019.300 | 0.999 | 505352720.800 | 0.99 | -1.289 | 0.923 | 2.509 | 0.87 |
| In situ component | 0.495 | 0.023 | 0.5 | 0.034 | 2.476 | 0.409 | -0.630 | 0.85 |
| Grade | 1.037 | 0.872 | 0.960 | 0.85 | 0.891 | 0.702 | 4.741 | 0.07 |
| Presence of necrosis | 0.890 | 0.770 | 1.319 | 0.46 | -4.612 | 0.252 | -5.418 | 0.23 |
| Tumor size (cm) | 1.384 | 0.000 | 1.361 | 0.000 | 0.335 | 0.622 | 0.648 | 0.39 |
| Insufficient axillary staging (>10 LNs) | 1.333 | 0.335 | 1.437 | 0.24 | 1.244 | 0.680 | 4.602 | 0.18 |
| Total number of resected LNs | 1.049 | 0.088 | 1.044 | 0.13 | 0.332 | 0.298 | 0.868 | 0.38 |
| Number of involved LNs | 1.183 | 0.000 | 1.112 | 0.01 | 0.459 | 0.327 | 0.666 | 0.18 |
| T tumor | 2.562 | 0.000 | 2.568 | 0.000 | 0.579 | 0.743 | 2.281 | 0.24 |
| N tumor | 2.393 | 0.000 | 2.008 | 0.000 | 3.832 | 0.052 | 4.563 | 0.03 |
| TNM staging | 4.471 | 0.000 | 3.936 | 0.000 | 5.812 | 0.049 | 3.827 | 0.14 |
| ER status | 0.726 | 0.318 | 0.650 | 0.18 | 5.406 | 0.111 | 8.455 | 0.03 |
| PR status | 0.658 | 0.158 | 0.736 | 0.31 | 7.789 | 0.012 | 9.309 | 0.009 |
| Hormone receptor status | 0.635 | 0 .161 | 0.577 | 0.09 | 5.470 | 0.115 | 8.325 | 0.03 |
| HER2-Enriched | 1.352 | 0.332 | 1.335 | 0.36 | -4.349 | 0.184 | -6.270 | 0.09 |
| Marker of proliferation Ki-67 | 1.020 | 0.004 | 1.02 | 0.005 | -0.091 | 0.204 | -0.052 | 0.53 |
| Type of breast surgery | 0.247 | 0.000 | 0.230 | 0.000 | -6.443 | 0.028 | -11.339 | 0.001 |
| Type of lymph node surgery | 1.362 | 0.462 | 1.576 | 0.33 | 4.298 | 0.296 | 6.299 | 0.19 |
| Chemotherapy approach | 3.157 | 0.005 | 4.212 | 0.000 | -6.747 | 0.137 | -5.199 | 0.21 |
| Chemotherapy regimen | 0.819 | 0.095 | 0.850 | 0.18 | -3.216 | 0.001 | -3.9 | 0.001 |
| Trastuzumab administration | 1.57 | 0.161 | 1.555 | 0.18 | -3.709 | 0.286 | -4.095 | 0.3 |
| Hormone therapy | 0.657 | 0.194 | 0.595 | 0.11 | 5.092 | 0.140 | 8.019 | 0.04 |
| Type of hormone Tx | 0.709 | 0.379 | 0.963 | 0.92 | -6.248 | 0.120 | -9.761 | 0.03 |
| RT field | 0.995 | 0.020 | 0.99 | 0.007 | 0.028 | 0.158 | 0.015 | 0.54 |
The Results of Univariate Regression Analysis for Predicting Disease Recurrence
5. Discussion
The findings of this study indicated that age, disease grade, lymphovascular/perineural invasion, the presence of necrosis, the type of axillary lymph node surgery, and hormone therapy did not have a discernible influence on the overall survival of breast cancer patients. Instead, overall survival was associated with disease stage, tumor molecular subtype, and chemotherapy regimen. Furthermore, our study revealed a correlation between disease-free survival and receiving MRM or BCS.
Several studies have highlighted increasing age as one of the most critical risk factors for the spread of breast cancer. In Iran, research reports have indicated average cancer patient ages of 46.8, 48.4, 47.0, 46.8, and 49.0 years, respectively (20, 21, 23-25). In our study, the average age was 49.35 years (age range: 36 - 54 years), with 65.1% of patients falling into the perimenopausal age category. Breast cancer incidence typically rises with age in both men and women, peaking in the 70 to 74-year-old age group for women (22). Similar trends were reported at ages 63 and 57 among white and black Americans, respectively (26). However, our results suggest a significant number of Iranian females are diagnosed with breast cancer at a younger age, without the typical risk factors associated with the disease.
A study by Bahrami et al. found that in Iran, the average age at menarche was lower than in some developed European countries like Switzerland and Sweden, higher than in Greece and Italy, and comparable to values observed in the USA and Colombia. Changes in children's habits and diet are likely responsible for the lower average age of menarche observed in Iran (27).
Sant et al. have highlighted geographical differences as one of the factors influencing the incidence of breast cancer. Despite varying incidence levels across countries, such as Spain and Italy (with the lowest incidence) and the Netherlands, Denmark, Finland, Sweden, and France (with the highest incidence), the most effective public health interventions seem to be those promoting lifestyle changes to reduce risk (28). Various studies have suggested reasons for treatment delay, including fear of mastectomy, lack of awareness, reliance on spirituality and herbal remedies, low education levels, and poor economic status (29-31).
Recent studies indicate an increase in the 5-year survival rate for breast cancer patients in Iran, possibly due to greater awareness and improved treatments (21, 24, 32). However, with a lower survival rate of 8% compared to American and European statistics, researchers have stressed the need for a comprehensive plan to address the rising incidence of breast cancer in Iran (21, 33). Additionally, many older patients have comorbidities like diabetes, coronary heart disease, hypertension, stroke, asthma, and chronic gastritis, which independently increase mortality risk. Nonetheless, advancements in surgical techniques now enable a larger number of these patients to achieve curative outcomes (34).
One aspect investigated in this study was examining factors predicting recurrence changes in breast cancer patients. Studies indicate that women with ductal carcinoma in situ face a higher risk of cancer recurrence, although the chance remains less than 30%. Recurrence often occurs 5 to 10 years after the initial diagnosis, and there's an increased risk of new breast cancer in the opposite breast. The choice of treatment affects the risk of recurrence, with a lumpectomy having a 25% - 35% recurrence rate for ductal carcinoma in situ, a risk reduced by 15% with the addition of radiation therapy. Currently, over 100% of women with ductal carcinoma in situ survive (35).
Mousavi et al. conducted a study in Tehran on breast cancer patients' survival, recurrence, and prognostic variables, revealing that patients with lymph node involvement had a higher probability of passing away or experiencing a recurrence of breast cancer (36). Similarly, our results indicated local or distant recurrence in 28% of patients, with 2.9% experiencing recurrence in previous surgery or regional lymph nodes.
Survival in breast cancer patients is influenced by various factors, with one of the strongest predictors being the disease stage at diagnosis. As observed in this study, death rates increase with advancing disease stages, resulting in decreased survival time for patients. Additionally, the overall survival outcomes of patients and their disease stage showed statistical significance. Recommendations regarding the delay of breast cancer treatment first emerged over a century ago (37). According to Bleicher et al., there is limited consensus regarding the impact of delay on survival in the United States, with research indicating that certain factors become more prevalent with increasing preoperative delays, although no single dataset can pinpoint all causes of delay (38, 39). In a study by Iqbal et al., race and ethnicity varied significantly among US women diagnosed with invasive breast cancer (40).
In the molecular subgroup classification of patients, our results indicated that patients with luminal A and luminal B subgroups had better survival compared to other subgroups, such as HER-2-positive and triple-negative breast cancer. Notably, a study by Hennigs et al. found that luminal A-like tumors (44.7%) were the most common, while triple-negative tumors (12.3%) had the worst outcomes (41). This suggests the potential for more tailored and effective treatments for each individual. Gong et al. also suggested that understanding the molecular subtype could lead to more personalized treatment strategies for metastatic breast cancer patients (42). The researchers proposed that lymph node metastasis, distant metastasis, and the triple-negative behavior of tumors arising from intrinsic biological differences could largely account for these statistical differences (40). Therefore, promoting knowledge and education for women, along with regular breast examinations, are crucial for early detection of breast cancer, potentially reducing mortality rates.
Mahmood et al. identified various factors affecting breast cancer patients' overall survival, including tumor size, lymph node metastases, receptor status, Her2neu positivity, skin involvement, and chest wall involvement (43). Meshkat et al.'s findings suggested that reducing tumor size increased the treatment rate from 68% to 76.3%. The recovery rate in T1 patients was approximately three times higher compared to T3+ patients, while it was reported to be 68% higher in T2 patients than in T3+ cases (21). However, other research has indicated conflicting results regarding the impact of tumor size on survival (20, 24). According to our findings, the median size of the initial breast tumor was 1.3 cm, with an average of 8 lymph nodes resected. Nevertheless, in 63 patients who underwent ALND, no lymph nodes were observed in the pathology report. Conversely, only 38.2% of patients who underwent ALND had at least 10 lymph nodes observed. This suggests that the number of lymph nodes examined in most patients is insufficient for proper axillary staging, thus limiting the validity of our investigation in this area.
Another aspect of our study focused on examining the malignancy grade and its impact on overall patient survival. The results revealed that the majority of patients had grades two and three, indicating high-grade disease with poorer differentiation. However, no significant difference was observed between disease grade and overall survival. Notably, the type of breast surgery significantly influenced the overall survival of breast cancer patients. Our findings indicated that the type of surgery plays a crucial role in patients' overall survival. According to research by Giovannelli, patients undergoing mastectomy surgery had 1.631 times lower survival rates than those undergoing BCS (44). Studies have highlighted non-physiological factors affecting patients who have undergone breast removal surgery, such as distress in partner relationships, concerns about body image, and feelings of depression, which contribute to various adverse psychological effects on patients (45).
Similarly, a study conducted in the Netherlands involving 17 3797 patients underscored the importance of surgery for survival. Despite accounting for stage, age, and adjuvant treatment, breast-conserving therapy offered a better prognosis than mastectomy (46). Comparable results have been reported in studies conducted in Iran, where researchers found that the mortality rate in patients undergoing MRM was twice as high as in those undergoing BCS. Moreover, the recovery rates of patients undergoing BCS and MRM were almost similar, with no significant difference observed in this regard (21). Another study by Baghestani indicated that patients undergoing MRM survived for more than 20 months, but their mortality rate was higher compared to those who underwent BCS surgery (24).
In our study, despite the majority of lesions being in the early stage of the disease, a significant number of patients underwent MRM and ALND. However, the performance of ALND and the number of lymph nodes examined to determine the N stage were insufficient in many patients, leading to inaccurate estimation and ultimately affecting treatment choices. Hence, it is imperative to provide comprehensive diagnostic and treatment facilities in healthcare settings, coupled with scientific updates in treatment services, to enhance treatment outcomes and improve survival rates.
The findings of this study provide valuable insights for health managers to develop long-term plans and appropriate treatment strategies to anticipate patients' conditions. However, the present study has some limitations. One limitation is the possibility of human errors when entering patients' data. To mitigate this limitation, a regression test was conducted to minimize its impact on the study's findings, given the retrospective nature and the potential for intervention variables that could influence them.
5.1. Conclusions
According to the findings of this study, the tumor molecular subtype, disease stage, type of chemotherapy administered, and the type of surgery undergone by patients all directly impact the overall survival of breast cancer patients. Furthermore, the results indicate that the chemotherapy approach, T and N tumor types, Ki-67, and RT field are significant variables in predicting changes in recurrence rates and mortality. Additionally, the history of breast cancer and chemotherapy regimen were identified as predictive factors for disease-free survival among the patients included in this study.