1. Background
Dual malignancies are rising in clinical oncological practice, regardless of the influence of geography and environment, which raises the possibility of genetic predisposition or factors related to oncological management. In 1860, Billroth was the first one who documented different primary cancers of multiple histologies in different organs at various intervals of time in a patient (1). The International Association of Cancer Registries and the International Agency for Research on Cancer (IACR/IARC), as well as the Surveillance Epidemiology and End Results (SEER), recommend the two most common definitions for multiple primary cancers. The SEER database recommends a period of 2 months in order to discern between metachronous and synchronous multiple primary cancers. IARC defines tumors as synchronous when diagnosed for a period of less than six months (or metachronous if more than six months) and occurring in different sites (2, 3).
The reported incidence of multiple malignancies ranges from 2–17% (2). In the SEER analysis (1973 - 1999) of 2.7 million cases, the second cancer was reported in 10% of the population in a different organ with a different histology. The likelihood of developing the third or fourth primary malignant tumors is between 2 and 12% in patients with two synchronous or metachronous tumors. According to meta-analyses, the likelihood of the second tumor is between 3 and 5%, including 0.5% for the third tumor and 0.3% for the fourth tumor (1).
The primary tumor itself, persistent environmental carcinogenic influence, chemotherapy, radiation therapy, field cancerization (Head and Neck), genetic predisposition, hormonal therapy, targeted therapy, immune suppression, ozone depletion, ongoing exposure to ionizing radiation, and rising frequency of organ transplant are the etiological factors implicating dual malignancy. Longer cancer patients’ survival due to recent therapy options may eventually result in the formation of a metachronous new primary, which may, in part, be caused by the treatment of an earlier malignancy (1). Modernized diagnostic techniques, such as Positron Emission Tomography (PET), which can detect indolent tumors, as well as improved cancer screening and patient surveillance, such as colorectal and breast cancer screening, are factors that can be attributed to the rise in the trend of multiple primary cancers (2, 4, 5).
Two primary malignancies are diagnosed using the Warren and Gates criteria (Box 1) (6, 7).
| Variables |
|---|
| 1. Histological confirmation of malignancy in both index and second tumors |
| 2. There should be at least 2 cm of normal mucosa between the tumors. If the tumors are in the same location, then they should be separated by at least five years |
| 3. The probability of one being the metastasis of the other must be excluded |
Warren and Gates Criteria for Diagnosis of Dual Primary Malignancies
Another classification for multiple primary cancers and multi-centric cancers was proposed by Moertel et al. as cited in Lv et al. in 1977, which is still applicable today (Table 1) (8).
| Groups | Details |
|---|---|
| Group I | Multiple primary cancers occurring in organs with the same histology |
| A | Cancers that occur in the same tissue and organ |
| B | Cancers that occur from the same tissue and different organs |
| C | Cancers that occur in bilateral organs |
| Group II | Multiple primary cancers that originate from different tissues |
| Group III | Cancers from different tissues and organs that concurrently exist with group I cancers, and they form multiple primary cancers of three or more cancers |
Classification of Multiple Primary Cancers a
The commonly seen synchronous and metachronous tumors are found in lymphoid, hematologic, breast, lung, bone, melanoma, thyroid, and soft tissue sarcomas. The risk areas for the patients who have received prior radiation treatment are the edges of the tumor bed and infield areas of radiation. As for the tumors related to chemotherapy, the sites of contact (aerodigestive mucosa), absorption (GIT), metabolism (liver), and excretion (kidney, lung) typically arise at these sites. The tumors tend to be more aggressive, with less nodal burden, and smaller in size, with higher and earlier metastasis in other organs. They are typically resistant to treatment interventions, which may be a result of the patient’s impaired physiological and immune system, chemo-radiotherapy-resistant gene activation, resistant clonal growth, and chemo-radiotherapy resistance (1).
It is crucial to establish the significance of dual malignancies due to the challenges they face in terms of determining strategies for screening, diagnosis, management, and prognosis of the disease. Second, synchronous malignancies in paired organs or the same organ are frequently difficult to diagnose, and only highly developed diagnostic tools, for example, molecular genetics, can be used to positively distinguish the tumors as two independent tumors. (1) When a patient has a prior history of cancer and has undergone treatment, it becomes difficult to diagnose a newly developed primary or additional metastasis. When two active malignancies are discovered in the same patient, the decision for the management that addresses cancer without increasing the toxicities or pertinent drug interactions, as well as not worsening the patient’s prognosis, is challenging. Moreover, during the inclusion of patients in clinical research protocols, most clinical trials do not include participants with a history of cancer or who are currently being treated for an active second malignancy (2).
2. Objectives
In this retrospective analysis, across a 5-year study period, we examined the pattern of occurrence of dual malignancies seen in our department at a single institute.
3. Methods
This study is a retrospective analysis of data of patients who presented with histologically proven synchronous or metachronous dual malignancy as defined by Warren and Gates criteria over 5 years, from January 2017 to December 2021, in the Department of Radiation Oncology. Patients had to meet the following requirements in order to be included in the study: having at least two neoplastic lesions confirmed by histological examination, with distinct histopathology in the two locations. Records with ambiguous information, such as those with unsure pathologic reports or no documentation of prior malignancies, were excluded, as well as the patients for whom the second malignancy had a suspicion of metastasis from the first primary malignancy.
The objectives of the study were to determine the demographic and clinical profile of patients with dual malignancies and the management received. We also tried to identify factors associated with the occurrence of dual malignancies.
4. Results
Over a 5-year period, a total of 9906 cancer patients were registered in the Radiation Oncology department of our institute, and 158 cases (1.6%) of multiple primary cancers were found among them. Among these cases, 57 (36%) were synchronous, and 101 (64%) were metachronous. The maximum period for the synchronous tumor occurrence was seen at 6 months (180 days). Metachronous tumor incidence intervals ranged from 1 to 15 years, with an average of 4.8 years. The shortest time difference of occurrence of the second malignancy was 3 months, where the index tumor was at the soft palate, larynx, and urinary bladder, and the second malignancy site was buccal mucosa, lung, and gall bladder, respectively. The longest time interval of occurrence was 13 years in a case of carcinoma larynx. The median age of diagnosis was 56 years, and the majority (64%) of patients were above 50 years old. Out of 158 patients, 73 (46.2%) were females, and 85 (53.8%) were males, making a 1.2: 1 male-to-female ratio. A family history of malignancy was found in only 9.4% of cases. A history of addiction in the form of smoking, tobacco chewing, and alcoholism was found in the records of 54 patients (34%). Table 2 shows the clinical characteristics of patients with synchronous and metachronous malignancies.
| Variables | Synchronous | Metachronous | Total |
|---|---|---|---|
| No. of patients | 57 (36) | 101 (64) | 158 |
| Median age (y) | 56 | 57 | - |
| Median interval of occurrence (y) | - | 3 | - |
| Age at diagnosis of primary tumor (y) | |||
| ≤ 50 | 20 (35) | 36 (36) | 56 (36) |
| > 50 | 37 (65) | 65 (64) | 102 (64) |
| Gender | |||
| Female | 30 (53) | 43 (43) | 73 (46.2) |
| Male | 27 (47) | 58 (57) | 85 (53.8) |
| Family history | |||
| No | 51 (89) | 92 (91) | 143 (90.6) |
| Yes | 6 (11) | 9 (9) | 15 (9.4) |
| Addiction | |||
| Smoking | - | - | 40 |
| Tobacco chewing | - | - | 16 |
| Alcohol | - | - | 10 |
Clinical Characteristics of Patients with Synchronous and Metachronous Malignancy a
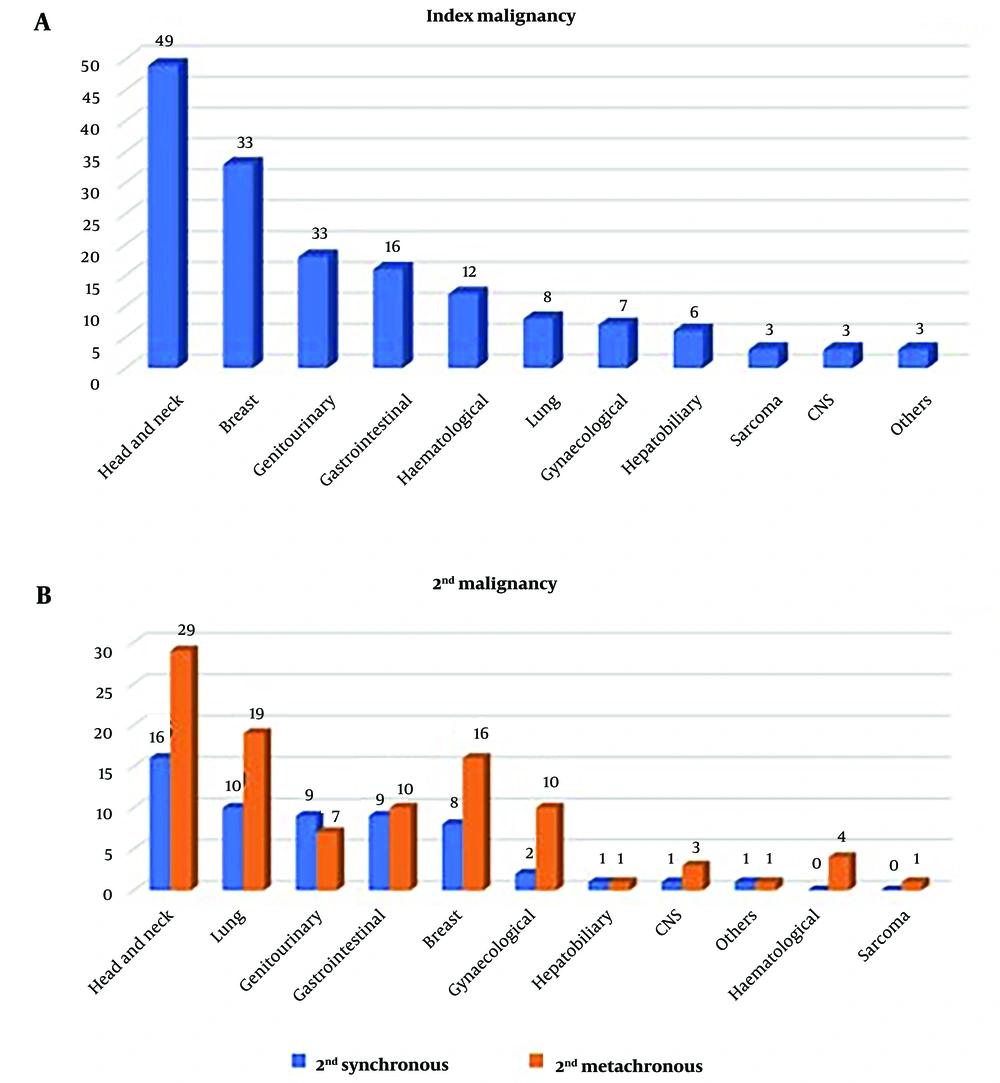
The most common site of primary tumor with dual malignancies was head and neck (49 cases; 31%), followed by breast cancer (21%) (Figure 1A). Among the second malignancy, the first two malignancies were found to be the same in both synchronous and metachronous groups. The most common site was the head and neck (28% of patients in synchronous and 29% in metachronous), followed by the lung (17.5% in synchronous malignancy and 19% in metachronous tumor) (Figure 1B) (Table 3).
| Malignancy Site | 1st Primary | 2nd Synchronous | 2nd Metachronous |
|---|---|---|---|
| Head and neck | 49 (31) | 16 (28) | 29 (29) |
| Breast | 33 (21) | 8 (14) | 16 (16) |
| Genitourinary | 18 (11) | 9 (16) | 7 (7) |
| Gastrointestinal | 16 (10) | 9 (16) | 10 (10) |
| Hematological | 12 (9) | 0 | 4 (4) |
| Lung | 8 (5) | 10 (17.5) | 19 (19) |
| Gynecological | 7 (4) | 2 (3.5) | 10 (10) |
| Hepatobiliary | 6 (3) | 1 (2) | 1 (1) |
| Sarcoma | 3 (2) | 0 | 1 (1) |
| CNS | 3 (2) | 1 (2) | 3 (3) |
| Others | 3 (2) | 1 (2) | 1 (1) |
Comparison of Site of the 1st Primary, 2nd Synchronous, and 2nd Metachronous Tumors a
Adenocarcinomas (40% of all instances with dual malignancy examined), squamous carcinoma (38%), hematopoietic and lymphoid tissues (7.5%), transitional cell carcinoma (6.3%), and sarcomas and soft tissue tumors (2.5%) were the most common pathology types (Table 4). Adenocarcinomas (54%) and squamous carcinomas (32%) were the most prevalent pathologic types among synchronous tumors, respectively. In metachronous tumors, the most frequent pathologic types differed, which were found to be squamous carcinomas (51%), followed by adenocarcinomas (36%).
| Histology Type | Index Malignancy (N = 158) | Synchronous (N = 57) | Metachronous (N = 101) |
|---|---|---|---|
| Adenocarcinoma | 63 (40) | 31 (54) | 36 (36) |
| Squamous cell carcinoma | 60 (38) | 18 (32) | 52 (51) |
| Transitional cell | 10 (6.3) | 3 (0.5) | 0 |
| Sarcoma | 4 (2.5) | 1 (0.2) | 1 |
| Hematological | 12 (7.5) | 2 (0.4) | 4 (0.3) |
| Other | 9 (5.7) | 2 (0.4) | 8 (0.7) |
Pathological Characteristics a
Of 157 index cases, 118 patients (80%) received radical treatment in the form of surgery, chemotherapy, and radiation. Palliative treatment was received in 9% of cases, whereas 11% defaulted for any further treatment. Treatment for the second malignancy was decided according to the performance status of the patients. Radical treatment was received in 46% of cases (82% metachronous, 18% synchronous), palliative treatment was considered in 42% of cases, and 22% of patients defaulted for further management.
5. Discussion
Patients with metachronous or synchronous malignancies are steadily rising, which poses significant diagnostic and therapeutic difficulty. Although the process of development of multiple primary cancers is still unknown, factors like the immune system of patients, their genetic susceptibility to carcinogens, and extensive exposure to carcinogens, such as chemotherapy and/or radiation therapy received during treatment, can be taken into consideration (4). There are factors that may lead to the prevalence of a second malignancy, such as genetic variables, environmental factors, diagnostic techniques, and follow-up information in various geographic regions (8). The incidence of dual malignancies in our study was 1.6%. Studies have reported an incidence rate of 0.73% to 11.7% in other countries (8-10). Vogt et al. reported an incidence of 2.4% to 8% of cancer patients have multiple primary cancers, and up to 17% do so within 20 years of follow-up (2).
The ratio of synchronous to metachronous malignancies differs in different studies. Metachronous tumors are more frequent than synchronous tumors, with a ratio of 2.7:1, according to the literature (11-13). Our study shows a ratio of 1.8: 1. We report the occurrence of 36% synchronous and 64% metachronous malignancies, which is similar to the study by Aydiner et al., who reported 34% synchronous malignancy compared to 66% metachronous malignancy (14). Flannery et al. reported 20% synchronous and 80% metachronous tumors in their series (15). A second primary malignancy can occur at any age. Various studies done so far have revealed that it is more common in old age. In this study, the median age of diagnosis was 56 years, and 64% were over 50 years of age. Sharma et al. reported a median age of 59 years at the time of the second tumor diagnosis, with 70% of patients above 50 years of age (16). Lv et al. reported that patients were older than 50 in 77.6% of cases (8). In various reviews of literature, the male/female ratio varies between 0.9 and 3.5, with male predominance (16, 17). Our results are consistent with previous studies, showing a male: female ratio of 1.2: 1.
Between the initial primary cancer and a second tumor, there may be a latent period of up to 15 years (1). In this study, a range of time of occurrence from 3 months to 13 years was seen, which is similar to the study by Chaudhary et al., who reported that the time to occurrence of a second malignancy was between 2 months and 17 years (18). The most common primary tumor associated with dual malignancy in our study is head and neck (31%), followed by breast (21%), which is similar to the study by Chaudhary et al. (18). Head and neck cancer is the most common associated synchronous and metachronous tumor, followed by lung cancer in both categories. However, there are discrepancies among various studies, which may be due to different geographical incidences. Sharma et al. reported carcinoma breast as the most common synchronous malignancy, and carcinoma breast and gynecological cancers were the most common metachronous malignancies (16). A study from Libya reported breast cancer as the most common synchronous tumor, while gastrointestinal cancer was the most common metachronous tumor (1). A study from China showed gastrointestinal and urogenital cancers are the most common synchronous and metachronous malignancies (8).
The probability of acquiring a second primary malignancy is 36% throughout the course of a patient’s lifetime for head and neck squamous cell cancer (4). Our study justifies this as head and neck cancers (31% of cases) were found to be the most common group who develop second malignancies, and they are also the most common synchronous and metachronous malignancies found. Among these head and neck cancer patients (31%), tobacco chewing and smoking were associated with 75% of the patients. The second-commonest malignancies that developed in these patients were head and neck cancer (49%), followed by lung cancer (29%). This can be linked to field carcinogenesis caused by exposure to well-known risk factors like smoking, using alcohol, and chewing tobacco. The mean duration of occurrence was 5 years.
The incidence of Dual malignancy in patients with breast cancer is seen to be 4.1% to 16.4%, which occurs within 5 to 8 years. The increased incidence of second primary tumors such as breast, bone, thyroid, and lung cancer is also influenced by the late toxic effects of radiotherapy and chemotherapy (2). In patients with breast cancer as an index malignancy, 47% had contra-lateral breast cancer as a second malignancy, followed by lung and gynaecological cancers. The median time of occurrence was 5 years. All the patients were treated with chemotherapy and radiotherapy.
The therapy for patients with multiple primary cancers is rarely mentioned in the literature, aside from numerous papers on the prevalence of multiple primary cancers. The following clinical characteristics in the presence of synchronous or prior cancer history should alert clinicians to the possibility that a patient may develop a second primary tumor: (1) patients who have experienced a recurrence after being exposed to environmental toxins (like smoking); (2) atypical primary tumor metastatic spread or new metastases that develop several years after an initial cancer diagnosis; (3) high tumor burden compared to the load of tumor markers; (4) suspicion of solid or hematological cancer following prior chemotherapy or radiation treatment; (5) different standard uptake value (SUV) of lesions on PET-CT or suspicious lesion during staging or follow up (2). The use of alcohol and cigarettes in any form should be discouraged, along with the adoption of a healthy diet and regular exercise, as part of a preventive plan for the patients. There is currently insufficient data to support the use of antioxidants and beta carotene as chemo-preventive medicines to avoid developing second primary malignancies (4).
5.1. Conclusions
Even in complete clinical remission, the possibility of developing a second malignancy should be considered in every cancer patient, and they must be closely monitored throughout their entire lives. In this regard, a fundamental and common surveillance program needs to be developed. To address this unanswered question of a cause-and-effect connection between synchronous or metachronous cancers, numerous multi-focused and multi-dimensional investigations are necessary. For treating these entities, no approved treatment plans have yet been suggested. In situations where there is a high probability that a second cancer would manifest, multidisciplinary therapy, a patient-specific strategy, and strict follow-up procedures should be taken into consideration.