1. Background
Microcalcifications are frequently linked to ductal carcinoma in situ (DCIS), with a prevalence of 50% to 75% in such cases (1-3). About 90% of DCIS instances manifest exclusively as mammographic microcalcifications, and if untreated, up to 40% of these lesions may advance to invasive disease. Given the significant risk, further diagnostic evaluations are essential (2, 4).
Presently, percutaneous biopsy or follow-up methods are utilized to assess these lesions further. The evaluation of mammographic microcalcifications adheres to the criteria established by the breast imaging reporting and data system (BI-RADS). Disease prevalence in BI-RADS 4-rated mammographic microcalcifications varies between 32% and 65.2%, and for BI-RADS 5-rated cases, it ranges from 91.4% to 100% (5). Histopathological examinations show positive predictive values (PPVs) for BI-RADS 4 lesions ranging from 20% to 65.2% (6-9). It is noteworthy that these values exhibit significant variability, covering data from various subcategories (BI-RADS 4a - 4c).
Given the lack of a reliable, established auxiliary screening tool to differentiate these lesions and considering the considerable risk of malignancy with follow-up alone, biopsies are generally recommended for nearly all patients with mammographic microcalcifications to exclude malignancy. The probability of biopsying a benign lesion in patients with BI-RADS 4-rated mammographic microcalcifications is approximately 34.8% to 62% (5).
In most scenarios, a biopsy is considered the gold standard for diagnostic evaluation. Nevertheless, there's a necessity to further refine the selection process for patients who truly need a biopsy. MRI has been employed to create decision support systems capable of predicting cancer recurrence, pathological complete response in patients receiving neoadjuvant chemotherapy, and conditions like Alzheimer's disease (10-15). These applications underline the potential of MRI in offering critical insights and supporting decision-making processes (5, 16).
The prevalence of disease in BI-RADS 4 lesions progressively increases from BI-RADS 4a to BI-RADS 4c categories. Various cutting-edge artificial intelligence methods have been investigated for detecting microcalcifications using different diagnostic tools such as mammograms.
2. Objectives
This study aims to determine whether MRI, in combination with computer-aided detection (CAD) software, can effectively help exclude malignancy in BI-RADS 4 lesions based on their specific BI-RADS 4 rating (a-c).
3. Methods
3.1. Study Design and Patient Population
In this single-center cross-sectional study conducted from August 2019 to June 2020, 40 patients with an average age of 47.75 ± 8.7 years were included. These patients underwent percutaneous or surgical biopsy for suspicious mammography-detected microcalcifications. Additionally, they received breast MRI within a timeframe of up to 8 weeks, with a median interval of 2 weeks (range 0 - 8 weeks) before the biopsy. The scheduling of MRI exams was based on the MRI facility's availability and the biopsy planning. The biopsies were typically scheduled for cases involving dense breast tissue, less well-defined masses, or multiple calcifications. Notably, high-risk patients were excluded from the study.
3.2. Imaging
At our institute, a full-field digital mammography system was used for imaging. Two radiologists with over three years of experience in breast imaging reviewed the microcalcifications and assigned them a BI-RADS category according to the BI-RADS guidelines. When it was not possible to definitively assign a BI-RADS category to recalled calcification lesions, a BI-RADS 0 designation was given. Additional views, such as spot compression views with or without magnification, were performed to assist in the final mammographic BI-RADS assignment.
For MRI, a 1.5 Tesla scanner was used, following the internationally recommended imaging protocol (17). The imaging protocol included T1-weighted short tau inversion recovery (STIR) and dynamic contrast-enhanced (DCE) sequences in the axial orientation. To enhance the MR images, intravenous administration of macrocyclic gadolinium-based contrast media was employed. The contrast media was administered in a single dose of 0.1 mmol per kg of body weight using automated injectors. Images were acquired in a pre-contrast phase, and the pre-contrast images were digitally subtracted from the post-contrast images to produce the DCE series.
To facilitate the analysis, a commercially available CAD system named CADstream was utilized. This system automatically highlighted areas of enhancement that exceeded a pre-set minimum threshold for initial enhancement by applying color overlays to all MRI slices. Additionally, CADstream enabled the assessment of the level of initial enhancement and differentiated between types of enhancement (persistent, plateau, and washout) in the late phase post-contrast injection, using color overlays.
To reduce bias and ensure readers did not remember details from the initial analysis, the CADstream readings were conducted six months after manually analyzing the same data set. All readers were proficient in applying BI-RADS in clinical practice. Separate BI-RADS-MRI score sheets were filled out by each reader for every lesion detected by MRI.
3.3. Data Analysis
MRI-breast radiologists with 3 - 10 years of experience performed all biopsies. Surgical interventions were carried out in cases where malignancy was confirmed. Board-certified breast pathologists possessing significant expertise conducted the histopathological examination of biopsy specimens. For lesions identified as benign in histopathological reports, follow-up mammography was scheduled for at least 24 months to confirm the benign nature of these lesions comprehensively. Certified radiologists who have been experienced in body and breast imaging for more than 3 years interpret all MR images. They had access to patients' medical histories and prior imaging, which assisted them in assigning an MRI BI-RADS category according to the BI-RADS lexicon criteria.
3.4. Statistical Analysis
The collected data underwent statistical analysis using IBM SPSS Statistics 25 (IBM Corp, Armonk, NY, USA) and Medcalc 17.8 (MedCalc Software, Ostend, Belgium). The evaluation of tumor extent, lymph node (LN) status, and multifocality in invasive breast carcinomas on breast imaging modalities was compared with actual pathological findings. The Kruskal-Wallis test was used to identify significant differences between various imaging modalities in assessing tumor extent. The chi-square test compared LN status as determined by multimodality breast imaging with final pathological results. Sensitivity, specificity, PPV, and negative predictive value (NPV) were computed. For breast MRI, sensitivity, specificity, PPV, and NPV were calculated with a 95% confidence interval based on BI-RADS category assignments (1 - 3: Benign vs. 4 - 5: Malignant) relative to the final histopathological diagnosis (benign vs. malignant). A significance threshold was set at P ≤ 0.05 to determine statistical significance.
4. Results
The histopathological examination identified a total of 40 lesions, with 22 categorized as benign and 18 as malignant, resulting in a malignancy prevalence rate of 45%. Among the malignant lesions, 11 were invasive ductal carcinomas, 3 were low-grade DCIS, and 4 were combinations of invasive ductal carcinoma and DCIS. The benign lesions included 5 cases of proliferative fibrocystic changes, 6 cases of sclerosing adenosis and duct ectasia, 10 cases of fibroadenoma/fibroadenomatoid hyperplasia, and one case of intraductal papilloma. MRI (MRI BI-RADS 4) successfully detected 15 out of 18 malignant pathologies as true positives. Out of the 22 benign findings, MRI accurately identified 13 as true negatives (6 MRI BI-RADS 2, 7 MRI BI-RADS 3), while 9 were incorrectly flagged as false positives (9 MRI BI-RADS 4) (Table 1).
| Variable | Pathology | Total | |
|---|---|---|---|
| Benign | Malignant | ||
| CAD (BI-RADS) | |||
| 2 | 6 | 0 | 6 |
| 3 | 7 | 3 | 10 |
| 4 | 2 | 0 | 2 |
| 4a | 4 | 6 | 10 |
| 4b | 3 | 6 | 9 |
| 4c | 0 | 3 | 3 |
| Total | 22 | 18 | 40 |
Number of Lesions Detected by MRI BI-RADS Compared to Histological Diagnosis
The findings yielded a sensitivity of 88.88% (95% CI: 51.7 - 99.7%), specificity of 69.56% (95% CI: 47 - 86.7%), PPV of 53.33% (95% CI: 37.1 - 68.8%), and NPV of 94.1% (95% CI: 71.1 - 99%). Utilizing CADstream for evaluating MRI findings indicated that all malignant pathologies were correctly classified as true positive, whereas only 10 benign findings were accurately marked as true negative, with 12 benign findings wrongly indicated as positive (Table 2). This resulted in a sensitivity of 100% (95%CI: 75.2 - 100%), a specificity of 50% (95%CI: 26 - 73.9%), a PPV of 59.09% (95%CI: 47.6 - 69.6%), and a NPV of 100%. In Figures 1, and 2, we present two examples of these patients.
| Variable | Pathology | Total | |
|---|---|---|---|
| Benign | Malignant | ||
| CAD (BI-RADS) | |||
| 2 | 5 | 0 | 5 |
| 3 | 5 | 0 | 5 |
| 4a | 7 | 3 | 10 |
| 4b | 1 | 6 | 7 |
| 4c | 4 | 5 | 9 |
| 5 | 0 | 4 | 4 |
| Total | 22 | 18 | 40 |
Number of Lesions Detected by CAD BI-RADS Compared to Histological Diagnosis
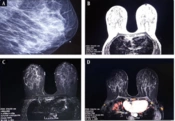
A, a 56-year-old woman with suspicious fine and pleomorphic microcalcification in the central of the left breast in mammography; B, T1-weighted image, axial view; C, T1-weighted images with fat saturation before contrast injection; D, T1-weighted image with fat saturation in first post-contrast time point MRI with using CADSTREAMsystem; E, post-contrast subtracted T1-weighted images at first time point with using CADSTREAMsystem without any pathologic parenchymal enhancement. The pathology study of the surgical specimen revealed moderate ductal hyperplasia (sclerosing adenosis).
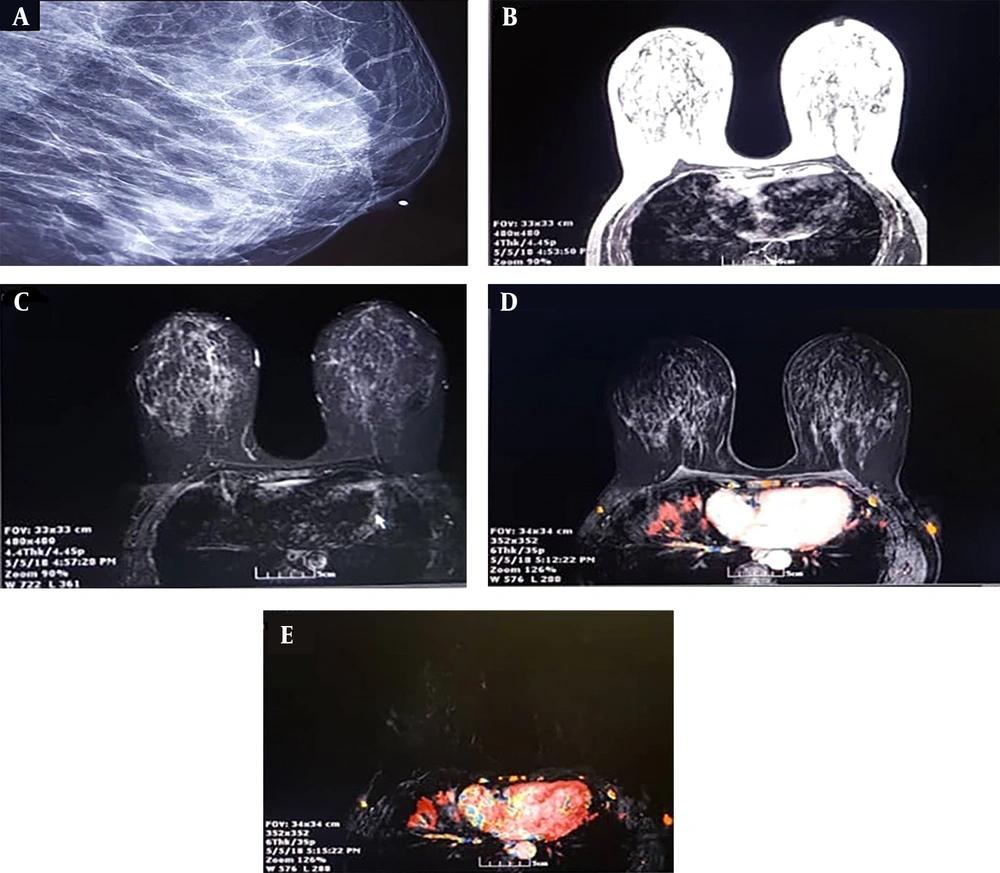
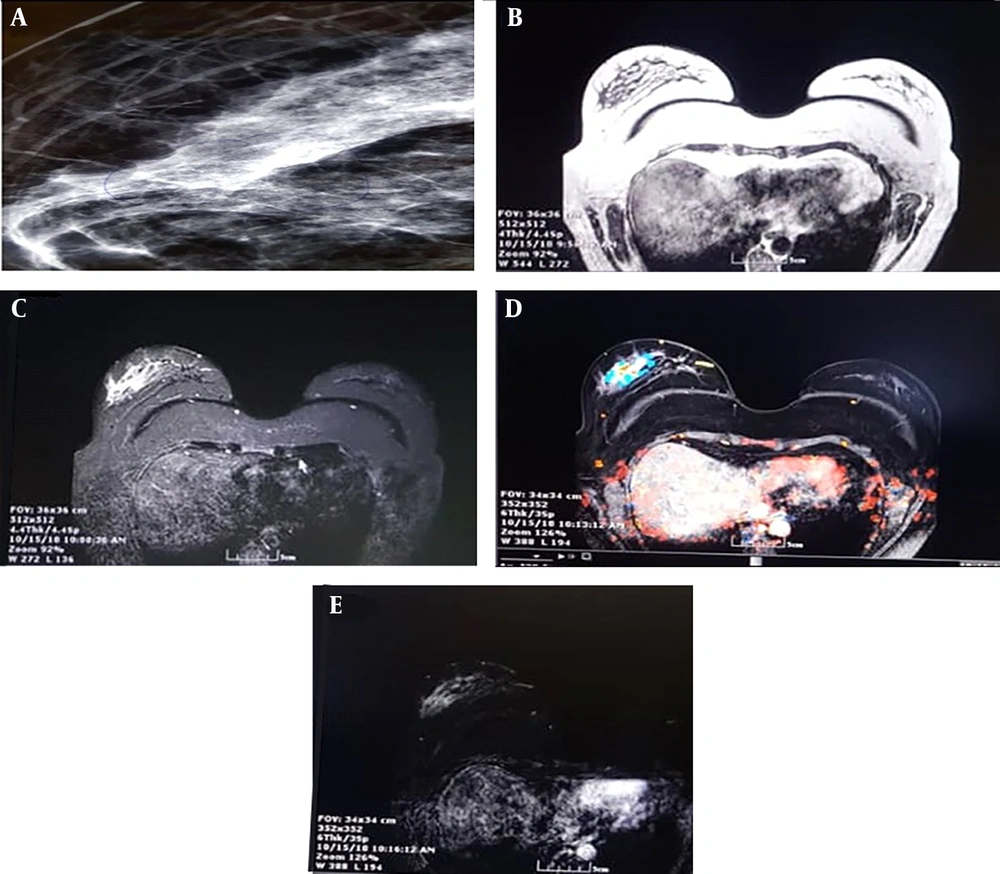
A, a 60-year-old woman with fine and pleomorphic microcalcification in the lateral of the right breast; B, T1-weighted image, axial view; C, T1-weighted images with fat saturation before contrast injection; D, post-contrast T1-weighted image at first time post with using CADSTREAMsystem shows heterogenous enhancement which in central part shows rapid focal enhancement with rapid washout with red color; E, post-contrast subtracted T1 weighted image at first time point shows focal enhancement in lateral of the right breast, The pathology study of surgical specimen revealed invasive ductal carcinoma with DCIS component.
5. Discussion
This study underscores the effectiveness of breast MRI in facilitating clinical decision-making for cases with mammographic microcalcifications, particularly those within the BI-RADS 4 category. Furthermore, the integration of CADstream can significantly improve MRI's diagnostic accuracy.
Our study demonstrated that breast MRIs are highly capable of identifying low-risk patients who are unlikely to have a malignant lesion, boasting a notable NPV of up to 94.1%. This significant NPV aids in avoiding unnecessary breast biopsies within our population, especially when BI-RADS 4 microcalcifications are present, and MRI findings are negative (BI-RADS < 4). Microcalcifications, often detected during screening mammograms, are frequently considered early signs of malignancy, underscoring the importance of these findings for patient care (2, 4).
MRI is regarded as the most effective method for identifying and diagnosing non-calcified breast cancer lesions due to its high sensitivity (5). However, the utility of MRI in evaluating mammographic microcalcifications remains uncertain. While contrast-enhanced MRI is not recommended for assessing microcalcifications directly, it excellently visualizes tissue vascularization through neoangiogenesis, offering the potential to identify both DCIS and invasive cancer associated with mammography-detected microcalcifications (18, 19). A recent meta-analysis advocated for the use of MRI to stratify malignancy risk in BI-RADS 4 mammographic microcalcifications (5), although the supporting data is limited, comprising only four studies with patient populations ranging from 27 to 78. This analysis suggested that MRI might help eliminate unnecessary breast biopsies when MRI results are negative (5). Cancer prevalence in these studies ranged from 32.1% to 62.5% (9, 20-22).
The data from a sample of 40 patients, including 18 individuals with BI-RADS 4 microcalcifications, indicated a cancer prevalence of 45%, aligning with previously reported values. The NPVs reported in prior studies ranged from 91% to 94%, consistent with our findings of a 94.1% NPV in BI-RADS 4 cases. These high NPVs indicate that breast MRI can accurately detect invasive cancers without missing any, potentially allowing for the postponement of planned biopsy procedures for mammographic microcalcifications without introducing adverse outcomes related to cancer (23). It is crucial to recognize that most DCIS cases are unlikely to progress to invasive cancer, leading to concerns about overdiagnosis and overtreatment (24).
The differentiation between biologically dormant cancers and active neovascularization is effectively highlighted by the presence or absence of contrast enhancement (25-27). Thus, breast MRI emerges as a critical tool in minimizing unnecessary biopsies for breast microcalcification cases, carrying minimal adverse effects like the potential oversight of isolated DCIS lesions. In our study, approximately 30.3% of lesions classified as probably benign were identified as foci (7 out of 22 patients), aligning with findings from other studies. Without breast MRI, these lesions would have necessitated unnecessary follow-up testing every six months over 24 months, as per our clinical protocol and the American College of Radiology's recommendations (28, 29).
In our patient cohort, the utilization of MRI eliminated the need for further biopsies. This indicates that a considerable number of procedures could potentially be avoided for BI-RADS 4 microcalcification cases, thereby maintaining a high standard of oncological safety. It is noteworthy that breast MRI can be expensive, and when no negative results or incidental suspicious lesions are found, an initial biopsy is advised. This approach, however, may lead to prolonged clinical workflows and potential delays in cancer treatment (30). Our study underscores the capability of MRI to reduce unnecessary biopsies for microcalcifications with a low risk of malignancy while also minimizing the delay in cancer treatment. A key strength of our study was the employment of CAD and the evaluation of its sensitivity and specificity in reducing unnecessary breast cancer biopsies.
Meeuwis et al. showed that CAD improved the specificity of MRI beyond manual analysis of enhancement, finding that automated analysis at 50% and 100% thresholds resulted in high sensitivity and specificity across readers with different experience levels (31). These results align with those of Kurz et al. and Meinel et al., who demonstrated that the performance of human readers in classifying breast lesions on MRI could be enhanced by a CADstream system incorporating lesion morphology and enhancement kinetics (32, 33). Furthermore, CADstream systems are instrumental in various tasks, such as assessing nodal status in breast cancer patients and detecting breast cancer recurrence (34, 35). Echoing recent research, our study revealed that using CADstream can augment human performance and reduce the incidence of unnecessary biopsies compared to MRI alone. Remarkably, in our research, all lesions with positive enhancement were confirmed as malignant, underscoring CADstream's potential to refine diagnostic precision.
Our study faces several limitations. Firstly, the relatively small sample size may hinder the definitive interpretation of the results. Secondly, the MRI evaluations were conducted by two radiologists. To minimize bias, having a single radiologist report the findings would be preferable. Thirdly, there is a potential for selection bias within the study. Future studies are recommended to involve larger sample sizes, extend over longer durations, and utilize a single radiologist for MRI reporting.
5.1. Conclusions
CAD systems serve as a supplemental tool rather than a substitute for radiologist reporting. This study underscores the significance of CAD in differentiating between benign and malignant breast lesions during 1.5-T MRI scans. Moreover, employing CADstream could help reduce interpretation variability among radiologists.