1. Introduction
Small-cell carcinoma, neuroendocrine type (SCCNET) is also known as Oat Cell carcinoma or poorly differentiated neuroendocrine carcinoma, and represents a rare histological finding in the head and neck area. This malignancy is even less frequent when it originates from nasopharynx tissues and, to our knowledge, there are currently only five cases reported in literature (1-5). This case report focuses on a multidisciplinary therapeutic approach and treatment options in a patient that was referred to our institution. Due to its known cellular aggressiveness and poor prognosis, this particular patient underwent a combined treatment modality that included radiotherapy (RT) and chemotherapy (ChT) in an attempt to control tumor growth and prevent metastatic spread.
2. Case Presentation
This was a 43-year-old female patient with no relevant medical history, namely tobacco or alcohol consumption habits. Patient complaints were progressive right hypoacusia and serous otitis media evolving over the last two years. In the last six months, the patient developed a right side and non-pulsatile tinnitus, persistent nasal blockage and frontal headaches. Tracing back her history, there were no records of epistaxis or systemic symptoms. Daily medication included oral contraceptives. The patient was referred to our institution for further investigation.
Otoscopy showed features of a right-side serous otitis media. There were no palpable enlarged lymph nodes. Nasofibroscopy revealed a tumor in the right lateral and posterior nasopharyngeal walls, obliterating the Eustachian tube. Inferiorly, the soft palate plane was preserved.
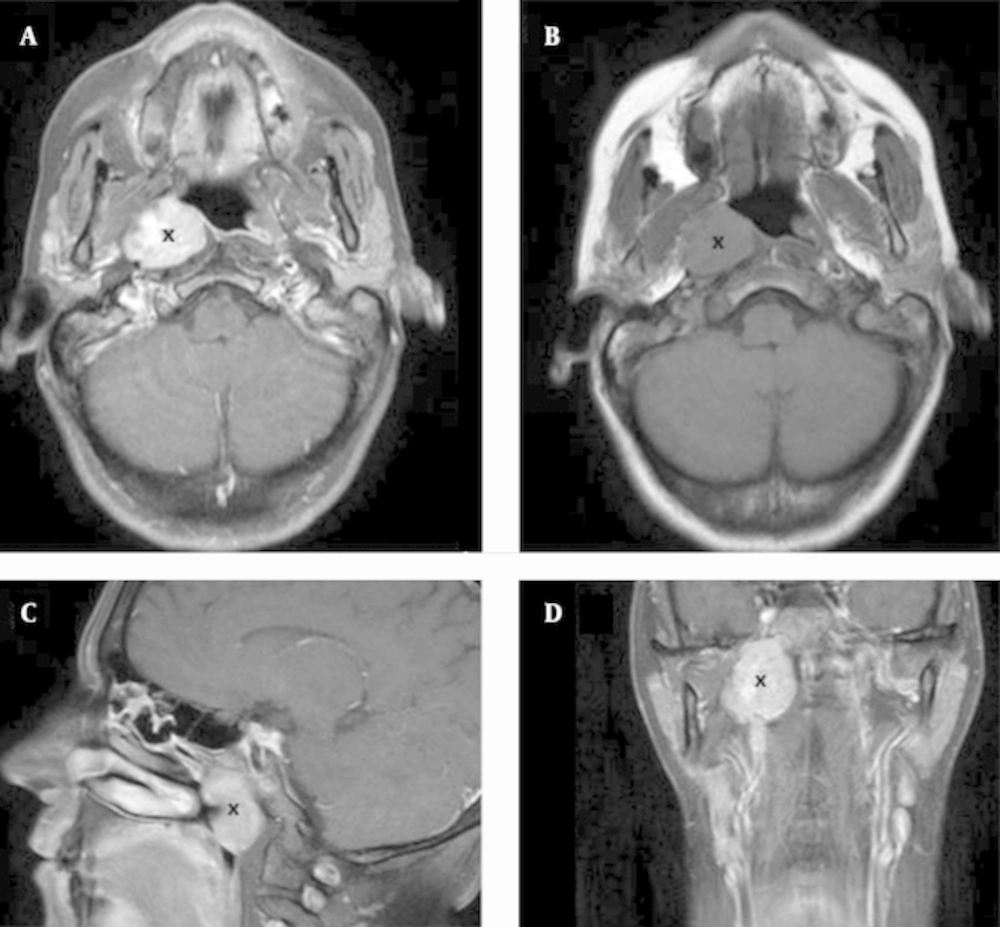
Computed tomography (CT) and magnetic resonance imaging (MRI) (Figure 1) of the paranasal sinuses showed an expansive lesion with regular and well defined limits (measuring 33 x 30 x 20 mm), centered in the right half of the nasopharynx and expanding to the retro and parapharyngeal spaces. The fossa of Rosenmuller and the torus tubarius were obliterated by the tumor. The lateral limit of the lesion contacted the internal carotid artery and the base of skull, but there were no observed signs of bone invasion.
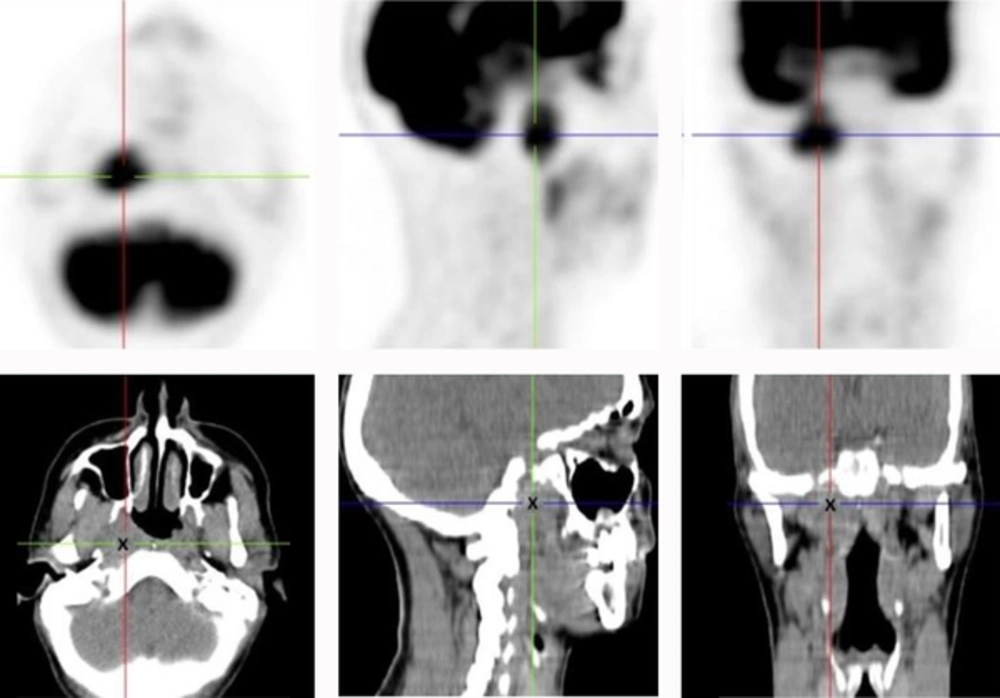
Positron emission tomography-CT (PET-CT) scan (Figure 2) revealed the same lesion with a maximum standardized uptake value (SUV) of 7.67. There was weak 18-fluorodeoxyglucose (18-FDG) uptake in some cervical lymph nodes, bilaterally, suggesting inflammatory activity. In addition, a systemic work-up with abdominal and pelvic CT-scans was unable to detect evidence of distant metastasis.
2.1. Pathology
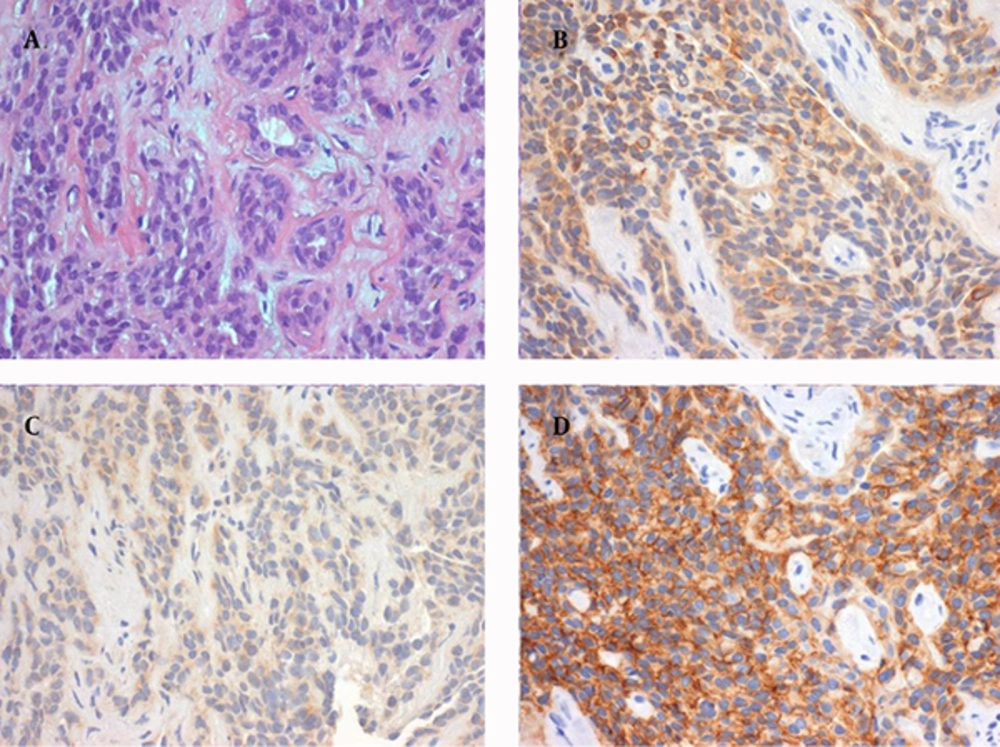
Tissue sampling of the lesion was obtained via endonasal microsurgery with biopsy of the nasopharynx. The pathology report revealed respiratory mucosa largely infiltrated by small, round and blue neoplastic cells as seen earlier in frozen sections. Immunohistochemical characteristics were consistent with a diagnosis of SCCNET, including positivity for cytokeratin 8,18, carcinoembryonic antigen (CEA), chromogranin A, synaptophysin (focal), CD56 (focal) and protein S100 (Figure 3) and negativity for glial fibrillary acidic protein (GFAP).
2.2. Treatment
Following a multidisciplinary team decision, the patient was assigned to treatment with ChT followed by RT in an attempt to control the disease. The originally planned 6 ChT cycles with monthly Etoposide and Cisplatin were administered as two induction cycles and two other cycles concomitant with RT. The last two cycles were eventually suspended due to toxicity.
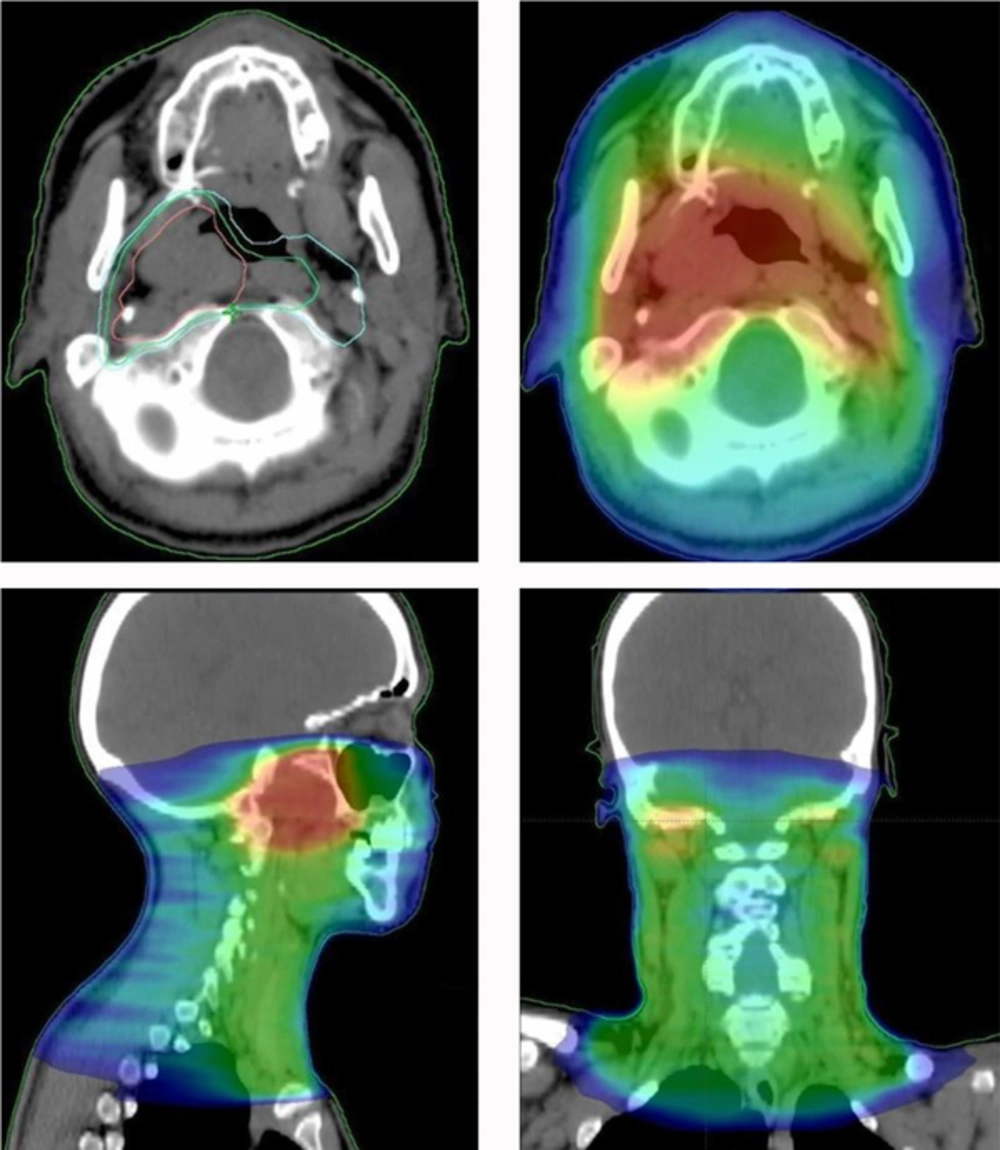
RT treatment consisted of a total dose of 70 Gy delivered in 2 Gy daily fractions, 5 times per week, with 6 MV photons and a volumetric modulated arc therapy (VMAT) technique (Figure 4). Three different volumes were designated according to the risk and extension of disease: Clinical Target Volume (CTV) 1: 50 Gy to the nasopharynx and bilateral cervical lymph node areas (levels II-IV) + 3 mm margin for subclinical spread; CTV 2: 66 Gy to the nasopharynx and retropharyngeal lymph nodes + 3 mm margin for subclinical spread; CTV 3: 70 Gy to the tumor + 3 mm margin for subclinical spread. PTVs (planning treatment volumes) were generated from the 3 CTVs, using 5 mm and 3 mm isotropic expansion from CTV 1/2 and CTV 3, respectively.
Regarding patient symptoms, PEG was removed at three months and grade 1 dysphagia persisted up to the eighth month re-evaluation; right hypoacusia remained unaltered and the skin of the neck area experienced residual pigmentation. Besides persistent grade 1 xerostomia, the patient progressively returned to her regular eating habits and regained weight. Concerning pain medication agents, the patient underwent progressive dose reduction, with successful replacement for a weak opioid (Tramadol) at the eighth month re-evaluation.
Treatment was suspended at the 21st RT fraction (5th week) for 6 days. The patient was admitted to the hospital due to severe nausea and vomiting unresponsive to oral antiemetic agents and grade 3 (common terminology criteria for adverse events-CTCAE 4.03) dysphagia with the need for percutaneous endoscopic gastrostomy (PEG). At this time patient also showed grade 2 mucositis of the oropharynx and grade 3 cervical dermatitis with the need for pain relief medication with Buprenorphine transdermal patches (78.75 μg/h). Another finding was grade 3 neutropenia and white blood cell count, as well as grade 3 fatigue.
2.3. Follow-up
Three months after treatment completion, re-evaluation MRI showed dimensional stability in the lesion (30 x 28 x 20 mm) that remained centered in the right fossa of Rosenmüller, and a post-obstructive opacification of the right middle ear. Bone scintigraphy, obtained at five months to exclude bone invasion, revealed an absence of bone lesions.
MRI and PET-CT performed at eight months documented unaltered lesion dimensions with a maximum SUV of 3.8. Facing no significant lesion downsizing and a positive 18-FDG uptake, 10 months after treatment completion, a biopsy to the initial mass was performed and revealed persistent disease.
3. Discussion
According to the world health organization (WHO) classification of head and neck tumors, SCCNET is a high-grade carcinoma with cell features that resemble small-cell carcinoma of pulmonary origin (6). Extrapulmonary small-cell carcinomas are uncommon and account for only 4% of all small-cell carcinomas, according to one study (7). When it derives from the sinonasal tract it is considered a rare tumor, but when it occurs in the nasopharynx it represents an even rarer situation. To our knowledge this is the sixth case described in the literature worldwide.
The neuroendocrine differentiation allows it to be stratified into one of two broad categories of neuroendocrine carcinomas, according to their epithelial (group 1) or neural (group 2) origin. The small-cell subtype is included in group 1 together with typical and atypical carcinoids, and is considered to be poorly differentiated (grade 3). Group 2 subtypes include paraganglioma, pheochromocytoma and neuroblastoma (8).
This particular histology is considered highly lethal and aggressive and the prognosis is poor. Age of incidence ranges from 26 to 77 years with a mean of 49 years. No sex, racial, or geographic predilection have been identified as well as any correlation with smoking or radiation exposure (6). Approximately 50% of the cases have cervical metastasis at presentation (9-11), and it is common to find distant metastasis at diagnosis due to its notable hematogeneous dissemination ability. The most common sites of spread are cervical lymph nodes, liver, lung and bone. Advanced tumors may invade the base of skull, orbit, or brain. Local recurrence is also frequent affecting about 45% of all patients (6).
Pulmonary small-cell carcinoma prognosis is dismal and the outcomes regarding extra-pulmonary disease are equally poor, with 5-year survival rates of 11 to 13% (12-15). Combined RT and ChT are considered to be the standard approach and surgery is reserved for limited stage initial disease or local relapse. With treatment, patients with extrapulmonary small-cell carcinoma have median survivals of 8 to 16 months (13-16). One study reported a median survival length for small-cell carcinomas of the head and neck region of 14.5 months with an estimated 5-year survival rate of 13% (12).
Therapeutic options are currently based on evidence regarding treatment of pulmonary small-cell carcinoma and range from surgery to isolated or combined RT and ChT (9, 10, 17). Interestingly, attempts have been tried to undergo curative-intent resections first, and the median survival for those who received surgical resection followed by adjuvant treatment was longer than those without resection (12-14, 18-22). However, no specific treatment guidelines are available due to the rarity of these tumors.
Regarding SCCNET of the nasopharynx, it is even more difficult to propose a standard treatment algorithm since only five cases have been reported to the best of our knowledge. Among these, three were treated with combined RT and ChT, one was treated with RT only and one was approached with surgery alone (1-5). Treatment specifications and follow-up times were either different or not specified, however, three cases eventually relapsed locally within the first 6 months and one of them (combined RT and ChT) experienced total remission with no detectable recurrence 9 months after treatment.
In our particular case, both 3- and 8-month re-evaluation MRI showed dimensional stability of the lesion, and this posed a real challenge in evaluating the true efficiency of the treatment strategy. PET-CT performed at 8 months after treatment completion delivered no clear information about tumor activity. While revealing hypermetabolism in the initial location, SUV was reduced to half of the pre-treatment values. Since there was no downsizing or remission, should this be considered a poor cellular response? The multidisciplinary team decision was to biopsy the lesion that eventually led to the diagnosis of persistent disease 10 months after treatment completion. Facing local treatment failure, the patient underwent salvage surgery. One could also address the question of whether prophylactic cranial irradiation (PCI) should have been provided. Interestingly, brain metastasis are less common in extrapulmonary small-cell carcinomas compared to small-cell lung cancer, so PCI’s known potential to improve survival and reduce symptomatic brain metastasis in small-cell lung cancer patients may not be valid to extrapulmonary disease (19). Its role in extrapulmonary small-cell carcinomas is unknown and there is currently insufficient data to suggest prophylactic cranial irradiation in such cases (12, 19).
In the end, this particular patient experienced severe acute toxicity effects attributed to combined treatment with RT and ChT that was ultimately unable to provide efficient local control of the primary disease. Many questions addressing the role, sequence and duration of combined therapy will remain unanswered until prospective trials with a large number of case series concerning SCCNET of various locations are conducted.