1. Introduction
Polymorphous low-grade adenocarcinoma (PLGA) is a rare malignant tumor which mostly develops in minor salivary glands (1). PLGA accounts for 7 - 11% of all benign and malignant neoplasms and about 19 - 26% of all malignancies in minor salivary glands (2). The palate is the most frequent location of PLGA. The appearance of PLGA in major salivary glands is extremely rare, and it has only been referenced in a few reports (3-5). A correct differential diagnosis is particularly important because treatment and prognosis are different.
2. Case Presentation
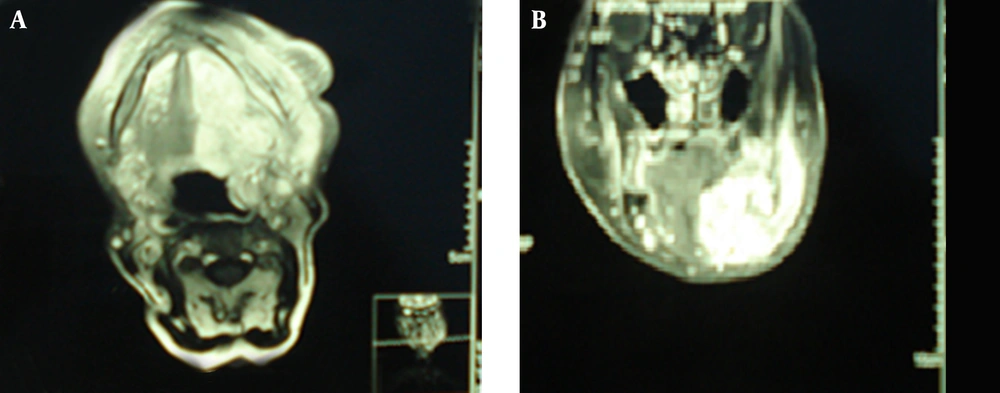
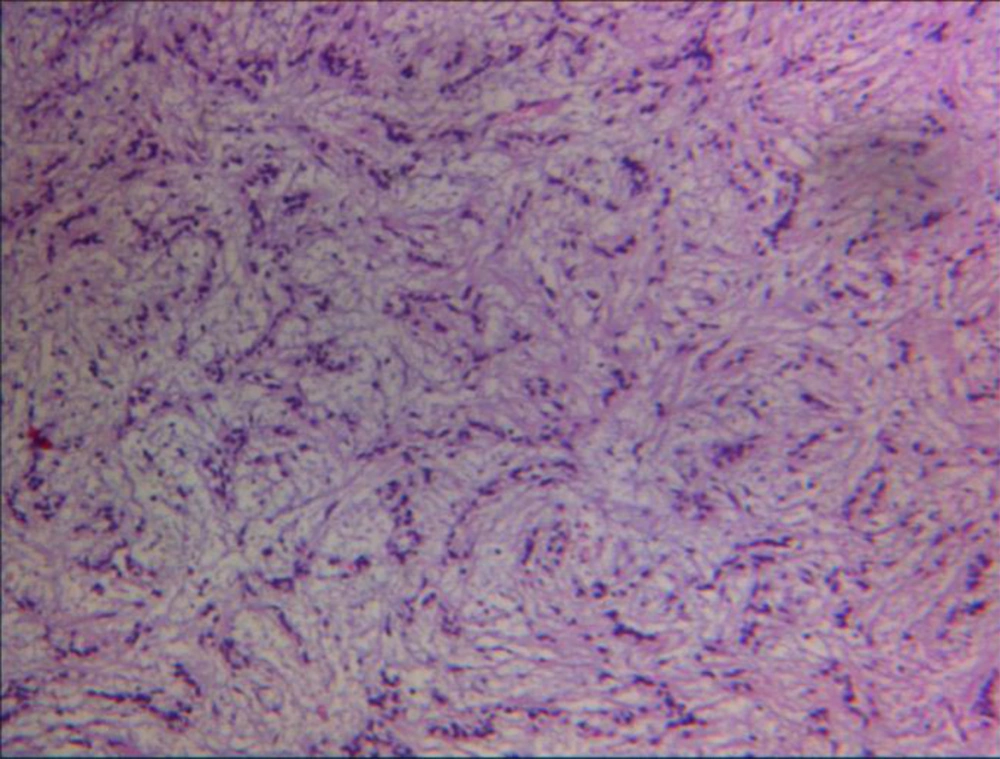
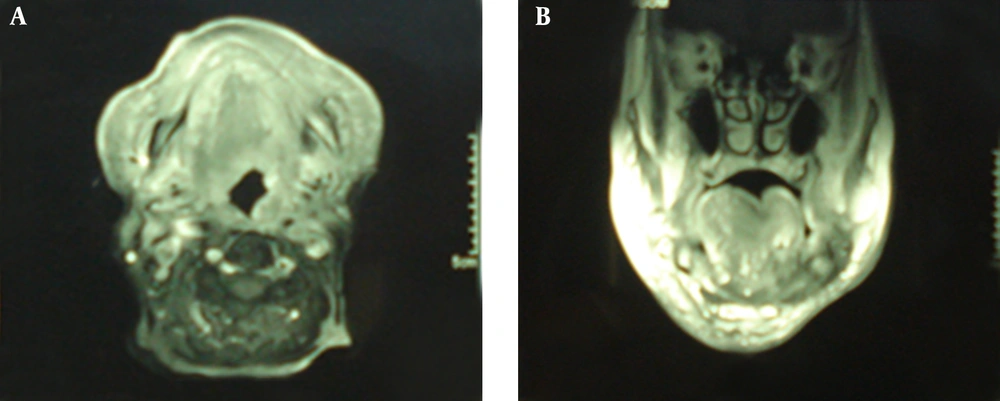
An 88-years-old woman residing in Iran was referred to the ENT Surgery Clinic of Shahid Sadoughi Hospital in April 2013 with a symptom of swelling in the left submandibular region. She had first noticed the swelling approximately 5 years earlier and it had gradually enlarged thereafter. On examination, a large firm and non-tender mass was palpable in the submandibular region. Neither lymphadenopathy nor enlargement of any other salivary glands was present. CT showed a well-defined mass with irregular enhancement in the submandibular gland (Figure 1). Magnetic resonance imaging (MRI) demonstrated that the mass had a low signal on T1-, a low to isosignal on T2-weighted images in the left submandibular region involved the left aspects of tongue, oropharynx, mandibular ramous and body, infratemporal fossa, maxillary bone, alveolar ridge, temporalis muscle and carotid space. After an injection of gadolinium the tumor showed marked non-homogeneous enhancement. The clinical diagnosis was suspected PA. An incisional biopsy was performed. Histopathologic examination revealed that tumor cells had mainly tubular patterns and consisted of isomorphic epithelial cells having round to oval nuclei with non-prominent nucleoli. Mitotic features were rare. Necrotic areas and perineural and vascular invasion were not observed. The tumor stroma showed myxoid changes with focal lymphocyte infiltration (Figure 3). The tumor was positive for AE1/AE3, vimentin and S-100 protein. CEA was partially positive. The Ki-67 labeling index was 20%. The pathologic diagnosis was polymorphous low-grade adenocarcinoma. Since the mass was very large and needed an extensive surgical procedure, the ENT surgeons decided to refer the patient for definitive radiation therapy. The patient treated with two parallel opposed lateral fields according to tumor volume showed by MRI and at least 2 cm margins from each side that finally became a 12 × 12 cm field with photon 6 MV. This field encompassed levels I and II and the upper part of level III of lymph nodes on the neck. After 4500 cGy the field shrank to 10 × 11 cm and the patient received more 2000 cGy to a total of 6500 cGy final dose. Physical examination 8 weeks later showed no evidence of previous mass and repeated MRI 12 weeks after termination of radiation therapy showed an excellent tumor response.
3. Discussion
First appearing in the second edition of the WHO Classification of Salivary Tumours in 1991 (6), PLGA was initially described in 1983 by Batsakis et al. and Freedman et al. (7, 8). Only pleomorphic adenoma and mucoepidermoid carcinoma are more common than polymorphous low-grade adenocarcinoma, and it is twice as frequent as adenoid cystic carcinoma (9). It has been reported to exhibit a nearly 2:1 female-to-male ratio and a wide age range (16 - 94 years) with a mean of 59 years (10). Our patient was an 88 years old woman. PLGA is generally an asymptomatic, slowly growing mass or swelling of the palate, cheek, or upper lip. Multiple synchronous occurrences have been reported (11). The present case had first noticed the swelling approximately 5 years earlier, and it had gradually enlarged thereafter. Most PLGAs will not cause diagnostic difficulty if careful attention is paid to the lack of pleomorphism and arrangement of the tumor cells. However, occasional tumors may be confused with adenoid cystic carcinoma or a cellular mixed tumor. The diversity of growth patterns and lack of dark-staining tumor cells support the diagnosis of PLGA and usually allow separation from adenoid cystic carcinoma, which has tubular, cribriform, and solid growth patterns. The majority of mixed tumors will have at least a focal mesenchyme-like component consisting of cartilage or a myxoid background or both, and foci with plasmacytoicl myoepithelial cells, allowing proper classification (12). Surgical resection with an appropriate safety margin is the treatment of choice. Elective neck dissection is not warranted unless the patient shows clinical evidence of lymph node metastasis. Historically, radiotherapy has been recommended for PLGA with inadequate margins, perineural or perivascular spread, and/or lymph node involvement (13). In our case surgery was impossible, but the patient felt discomfort and heaviness in the left side of her face and insisted on receiving some kind of treatment. Therefore, we decided to try radiation therapy. Castle et al. (14) reviewed 164 cases of PLGA and in that group 17 patients received radiation therapy after incisional or excisional biopsy or wide excision. The patients who had received radiation therapy were more likely to show evidence of disease at their last follow-up; however, the overall survival rate was not statistically different from those who did not receive radiation. In another case report Makita et al. (15) reported a patient with PLGA of palate who refused surgery and was instead treated with intra-arterial cisplatin and proton beam therapy and showed disease control. Uemaetomari et al. (16) presented two cases of PLGA who received post-operative radiation and concluded that radiation therapy possibly helps to improve local control. However, Evans (17) found no significant differences in the rates of regional metastases, distant metastasis, and patient survival according to the type of initial therapy or margin status. Since PLGA is uncommon, there is no clear recommendation for elective neck irradiation; however, as our patient had an advanced disease, we decided to treat at least levels I and II and upper part of level III. It should be noted that late recurrences, metastases, and fatal outcomes, though currently infrequent, may not be as rare as previously thought. There are some reports of transformation to a higher-grade tumor (18), and these patients have a less favorable clinical outcome. Since there can be protracted periods of time between initial resection and recurrence, a long-term follow-up is needed.