1. Background
Breast cancer (BC) is the most common type of malignancies in women and the third most common cancer after lung and gastric cancers all over the world (1). Most of the breast cancers are adenocarcinoma (about 95%) (2). Although the incidence of BC has increased from 85 in 100000 to 105 in 100000 between 1980 and 1989, the death rate of BC is not raised and its life expectancy is extended. This raise has no specific age range and might be associated with better screening approach and medical and surgical treatment (3).
Locally advanced breast cancer is a term that refers to most advanced-stage non-metastatic breast tumors and includes a wide variety of clinical scenarios. These tumors remain a difficult clinical problem as most of them will lead to breast-conserving surgery and probably relapse. Breast-conserving surgery is the first request of patients and an option for breast surgeons. One of the new treatment protocols for these patients is neoadjuvant therapy. With this method, the size and volume of breast mass will be reduced to facilitate resectioning (4). As the localization of the primary tumor site and the tumor bed is not easy in many cases, radiologic intervention and localization are needed (5-7).
Preoperative commercial radio-opaque markers and recently, the radioactive seed localization are the standard methods for tumor bed localization. Unfortunately, the gold standard method of localization, i.e. seed localization, is not available at all institutes and the commercial radio-opaque markers are relatively expensive (2). To the best of our knowledge, there are only two papers in the literature about the use of surgical clips instead of seed or commercial markers for tumor localization in breast cancer patients undergoing neoadjuvant chemotherapy (8, 9). Displacement of the surgical clip and its migration out of the lesion is the most worrisome and important consideration (10). In this study, we evaluated the accuracy of the radio-opaque surgical clip marker and wire localization in advanced breast cancer in 35 patients based on both radiological and pathological findings.
2. Methods
This cohort study was conducted at Omid Hospital, Mashhad University of Medical Sciences, a teaching tertiary hospital, between 2015 and 2017 (ethical committee code: IR.MUMS.fm.REC.1395.334).
35 patients older than 18 years with advanced breast cancer who were candidates for breast-conserving surgery after neoadjuvant chemotherapy were enrolled in the study after obtaining their informed consent. Exclusion criteria were pregnancy, breastfeeding, history of previous surgery, and patient dissatisfaction.
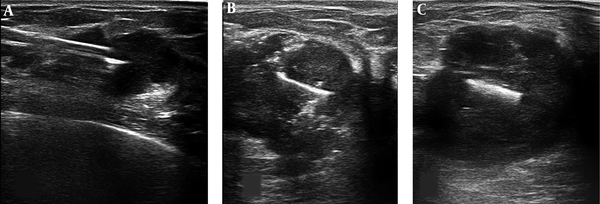
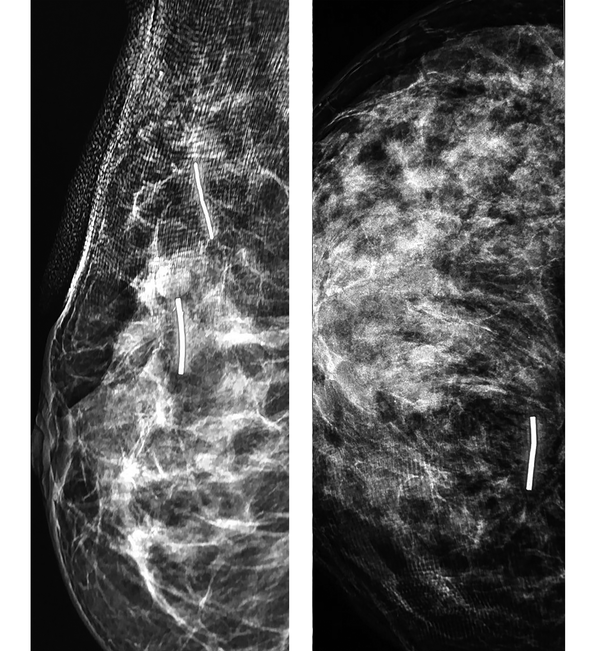
The localization of the lesion was performed by an interventional radiologist under ultrasound guidance. Before the start of neoadjuvant chemotherapy, the targeted ultrasound was performed for the exact localization and specification of the mass (axes of involvement, the nearest and the endmost distances of the nipple, satellite lesion, and nipple or axillary involvement). Afterward (Figure 1), a radio-opaque manually straightened surgical clip marker (VITAITEC, titanium hemostatic clips, 6 large) was inserted at the center of the mass by coaxial needle (MEDAX, a Coaxial needle for Bio-Feather, 14G × 160 mm). The largest size of the surgical marker was used for the better visualization during preoperative final wire localization. At the end of this step, after the exact evaluation of clip position inside the mass by targeted ultrasound, a single-view mammography was taken for the better evaluation of the marker location (Figure 2).
Ultrasound images of marker localization steps: A, Entrance of the coaxial needle into the center of the lesion. B, The straightened surgical clip was inserted into the center of the mass. Few air bubbles are also seen inside the mass that entered by stylet during clip insertion. C, The post clip insertion image of a breast mass.
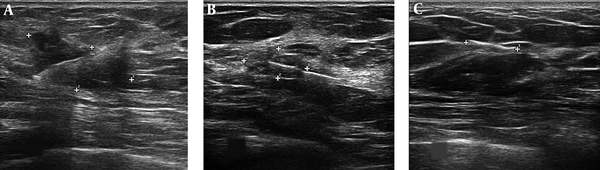
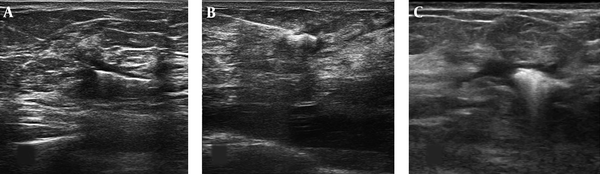
The neoadjuvant chemotherapy protocol was 4AC+4T-+Trastuzumabe. The time interval from the date of clip insertion and the start of neoadjuvant therapy to the date of surgery was 167 ± 26 days. After completing the courses of neoadjuvant chemotherapy, repeat targeted ultrasound was performed for the exact localization of clip alone (tumor bed) and/or probably residual mass to assess the response to treatment and tumor bed location (Figure 3). This localization was matched with clips location after neoadjuvant chemotherapy for the clips movement assessment. Then, a localization wire (TSK breast localization needle 20G, 100 mm) was introduced adjacent to the clips under ultrasound guidance (Figure 4A and 4B). In a few patients, radionuclide localization was performed exactly similar to radioactive occult lesion localization (ROLL) technique (Figure 4C). On the same day, an expert surgeon exposed and removed the bed of breast mass with regard to the extent of the tumor at the time of first diagnosis and wire location. The resected mass was assessed for marginal involvement and location of the clip relative to the bed tumor by an expert pathologist.
Data analysis was performed by SPSS version 16. A P value of less than 0.05 was considered as the significance level.
3. Results
In this study, 35 patients with breast carcinoma were enrolled. The evaluated variables are shown in Table 1. All were female with the mean age of 53 ± 9.3 years. In 30 patients (85.7%), a core needle biopsy showed invasive ductal carcinoma and others were lobular carcinomas. In 18 patients (51.4%), the right breast was involved. The most common site of the lesion was the upper-outer quadrant of the breast (15 cases, 42.9%). Satellite lesion was noted in 17.1% of the patients. The mean maximum diameter of the lesions was 3.8 ± 1.1 cm. After the completion of localization, the marker was seen at the center of the lesion in 32 (91.4%) patients and it was mistakenly inserted paracentrally in 3 patients.
| Variable | N | % |
|---|---|---|
| Breast side | ||
| Right | 18 | 51.4 |
| Left | 17 | 48.6 |
| Breast quadrant | ||
| Subareolar | 1 | 2.5 |
| Upper outer | 15 | 42.9 |
| Upper inner | 11 | 31.4 |
| Lower outer | 4 | 11.4 |
| Lower inner | 4 | 11.4 |
| Core needle biopsy | ||
| Ductal | 30 | 85.7 |
| Lobular | 5 | 14.3 |
| Placed marker in the mass before NAC | ||
| Central | 32 | 91.4 |
| Peripheral | 3 | 8.6 |
| Displacement of the marker inside the mass | ||
| + | 3 | 8.6 |
| - | 32 | 91.4 |
| Displacement of the marker outside of the mass | ||
| + | 0 | 0 |
| - | 35 | 100 |
| Satellite lesion | ||
| + | 6 | 17.1 |
| - | 29 | 82.9 |
| BIRADS | ||
| 4a | 3 | 8.6 |
| 4b | 10 | 28.6 |
| 4c | 6 | 17.1 |
| 5 | 29 | 82.9 |
| Detected marker in pathology specimen | ||
| + | 33 | 94.3 |
| - | 2 | 5.7 |
| Margin of specimen | ||
| Free | 35 | 100 |
| Invasion | 0 | 0 |
| Resection in the pathology report | ||
| + | 30 | 84.7 |
| - | 5 | 14.3 |
| Relapse | ||
| + | 0 | 0 |
| - | 35 | 100 |
Variables of the Study
After neoadjuvant chemotherapy, first, the surgical clip movement was evaluated sonographically. All patients had a response to adjuvant chemotherapy as a decrease in size in 22 patients (63%) and complete effacement of mass in 13 patients (37%). The mean maximum diameter of the residual lesion was 0.99 ± 0.98 cm.
In patients with residual mass after neoadjuvant chemotherapy, the marker was localized in the peripheral part of the residual mass in 6 patients while in the other 29 patients, the marker was localized in the central part of it. In 32 patients (91.4%), no marker displacement was detected and in 3 patients (8.6%), the marker displacement was found inside of the mass. In two out of the three patients, the marker was inserted at the center of the mass and later was moved to the periphery while in one patient, it was introduced initially to the periphery and then detected at the periphery of the tumor bed and adjacent to the normal breast tissue (Figure 3B). The clip migration out of the lesion or other adjacent axis or quadrant was not seen in any patient.
After the surgery, the average volume of the resected mass was 616.2 ± 442.7 millimeter square. In 10 (28.6%) patients, a complete response to adjuvant chemotherapy was seen at pathology. In all patients, tumor bed associated was resected in the pathology examination and marginal involvement was not seen in any of the cases. In the pathology report, 33 (94.3%) markers were detected and 2 (5.7%) markers were not detected in pathology samples (probably lost during surgery).
The accuracy of the surgical clip marker and wire localization was 100%. None of our patients experienced complications (infection or hematoma) after the procedure. After a 6 to 18-month clinical and ultrasonic follow-up, tumor relapse was not seen in any patient.
4. Discussion
Considering the worldwide high prevalence of breast cancer and more than one million new cases per year, screening and diagnostic methods are of utmost importance. Breast-conserving surgery after neoadjuvant chemotherapy is the first choice for patients with locally advanced breast cancer. This leads to the size reduction of the mass and facilitates surgical resection (11). Prerequisite of this protocol is the localization of original tumor bed and probably tumor remnant. The placement of tumor-marker clips is an integral part of this multidisciplinary approach, which is especially important for patients with complete effacement of mass after neoadjuvant chemotherapy. The complete sonographic effacement of the mass was observed in 13 of our patients (37%). In 10 (28.6%) patients, a complete pathologic response was seen.
Edeiken and Oh and their colleagues used ultrasound-guided implantation of metallic markers in the breast mass and concluded that this method effectively localizes the tumor bed after complete or near complete response to neoadjuvant chemotherapy (12, 13). In addition, the placement of seed on these patients before surgical resection is a safe and affordable method and has been associated with better surgical results (14). Seed localization is one of the methods that use radioactive-I 125; because of its longer half-life, this method can be performed before neoadjuvant therapy. Radioactive seed is placed in the breast mass with ultrasound guide before the neoadjuvant course and remains until the end of the treatment and surgery. Radioactive-I 125 is detected with a gamma probe during operation and tumor bed resection is performed.
The cost-effectiveness of these methods and their availability are very crucial. To eliminate the need for seed and commercial tissue markers, a surgical radio-opaque clips marker “known as Ligaclips” was introduced in two papers in the literature (8, 9).
Although commercial clip markers have a specific shape (two horns) to prevent displacement, surgical clip migration is very problematic (10). This is especially obvious in straightened surgical clip markers. In both the studies by Masroor et al. and Youn et al., surgical clip migration was not seen by imaging modalities (8, 9). Our results showed that surgical clip migration occurred inside the tumor in 8.6% of the patients. However, displacement to the outside of the tumor and or adjacent quadrant was not observed in any of the patients. In two out of three patients with marker displacement inside the lesion, the marker was inserted into the center of the mass that later moved to the periphery. In the other patient, the marker was introduced initially to the periphery of the lesion that was later detected to be at the periphery of the tumor bed and adjacent to the normal breast tissue.
As we used the largest size of surgical clip marker, it was easily detectable during preoperative final wire localization. Due to special circumstances, in some of the patients, radionuclide localization was performed exactly similar to radioactive occult lesion localization (ROLL) technique (15).
The pathologic evaluation of all 35 patients showed the tumor bed with free margins and in 33 (94.3%) patients, the marker was detected in the pathology specimen. This is another reason for the accuracy of the technique and non-significant migration of the clips. In 2 (5.7%) patients, the marker was not present in the pathology specimen, which may be attributed to being lost during or after the operation. Our results confirmed the claim of Masroor et al. and Youn et al. that surgical clip migration does not occur in patients with neoadjuvant chemotherapy (8, 9).
In this study, the accuracy of the surgical clip marker and wire localization in advanced breast cancer cases was 100% and it was without side effects.
As neoadjuvant chemotherapy has become more common, the placement of available cheap breast marker has been more important. The standard titanium surgical clips are inexpensive, available, and safe markers that are easily visualized on imaging modalities, do not interfere with medical treatment and future MRI examination, and can be removed during the surgery. In comparison with commercial localization markers, a surgical clip was inserted into the lesion via a co-axial needle without the need to use a special applicator.
The main limitation of this study was its performance in an academic center with limited patients, which can influence the accuracy of the test. We inserted the surgical clip into the center of the tumoral lesion that had a firm pathologic consistency. Its insertion to the periphery of the lesions with loose consistency may change the results, especially in case of hematoma formation. In addition, this study was performed in patients who underwent eight courses of neoadjuvant chemotherapy (approximately for 5 months (167 ± 26 days) while longer time regimens may change the results.
4.1. Conclusions
In the absence of seed localization, the combination of a surgical clip and wire localization is an easy, safe, available, and accurate choice for localizing tumor bed in advanced breast cancer patients who are candidates for neoadjuvant chemotherapy.
.jpg)