1. Background
Whole brain radiotherapy (WBRT) is an integral component in the management of brain metastasis (BM) (1, 2). Several studies have shown that, central nervous system tumor control is a strong predictor of neurocognitive decline than WBRT itself. Progression of brain metastasis is a much greater cause of neurocognitive dysfunction than WBRT alone (3). Even in studies combining WBRT and radiosurgery, tumor control was found to correlate with neurocognitive stability (4, 5).
2. Objectives
Our study was performed to correlate the changes in magnetic resonance spectroscopy from the hippocampal and perihippocampal areas and mini mental state examination after WBRT with various patient related, disease related and local tumor related factors to identify the patient cohort who may benefit from hippocampal avoidance WBRT.
3. Methods
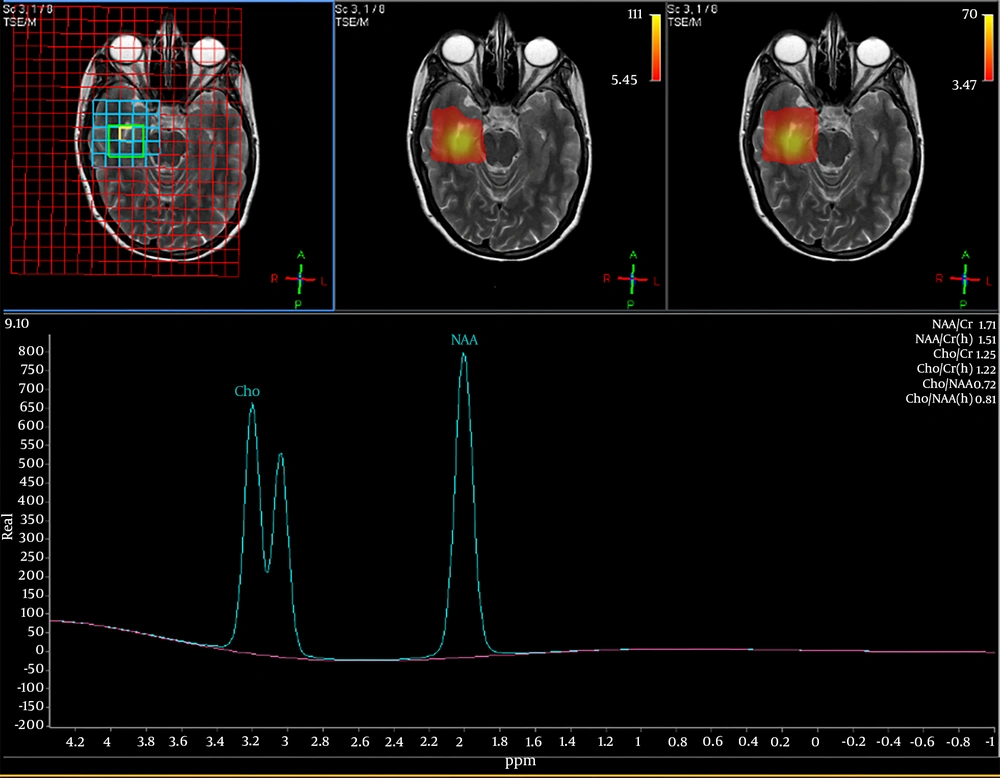
We enrolled 33 patients referred to department of radiotherapy for management of BM. After the initial clinical and neurological assessment of patients, they underwent volumetric magnetic resonance spectroscopy of the bilateral hippocampal and perihippocampal areas to measure the choline-NAA index (CNI) which indirectly estimates the anatomical integrity of the region. A CNI value of less 1 indicates that the level of N-acetyl aspartate, the neurotransmitter of normal brain is higher compared to choline which is a marker of cell proliferation and damage. The CNI values were taken in a volumetric manner from the bilateral hippocampal and perihippocampal areas. Figure 1 shows MR spectroscopy peaks and definition of CNI.
Mini mental state examination (MMSE) was evaluated for all the patients to assess their baseline neurocognitive function status. All the patients then underwent WBRT on linear accelerator to a dose of 30 Gy in 10 fractions. The MRS and MMSE along with clinical and neurological evaluation were performed at 1 month, 3 months, and 6 months post WBRT. The trend of these values were correlated with 13 clinical factors; patient related (5), systemic disease related (4) and local brain disease related factors (4).
The patient related factors were, age (< 50 or > 50 years), KPS at presentation (< 70 or > 70), RPA (class 2 or higher), duration between last therapy for primary diagnosis and diagnosis of BM (< 6 months or > 6 months) and presenting complaint of BM (with or without motor deficit/CN (cranial nerve) palsy). The primary disease related factors were site of primary (lung/breast vs. others), histology (adenoca vs. other), and modalities of therapy used for primary [with or without systemic long term chemotherapy (a chemotherapy schedule lasting for 6 months such as in breast cancer and lung cancer) with or without biological agents] and presence of extracranial disease (yes or no). The local disease related factors were site of BM (supra or infratententorial), number of metastasis (solitary/oligo vs. multiple), size of the largest lesion (< or > 4 cm) and proximity to any perihippocampal region (< or > 2 cm).
Variation in the means of repeated measures of MRS and MMSE was tested by ANOVA and the variation of the mean was correlated with each prognostic factor by univariate and multivariate analysis.
4. Results
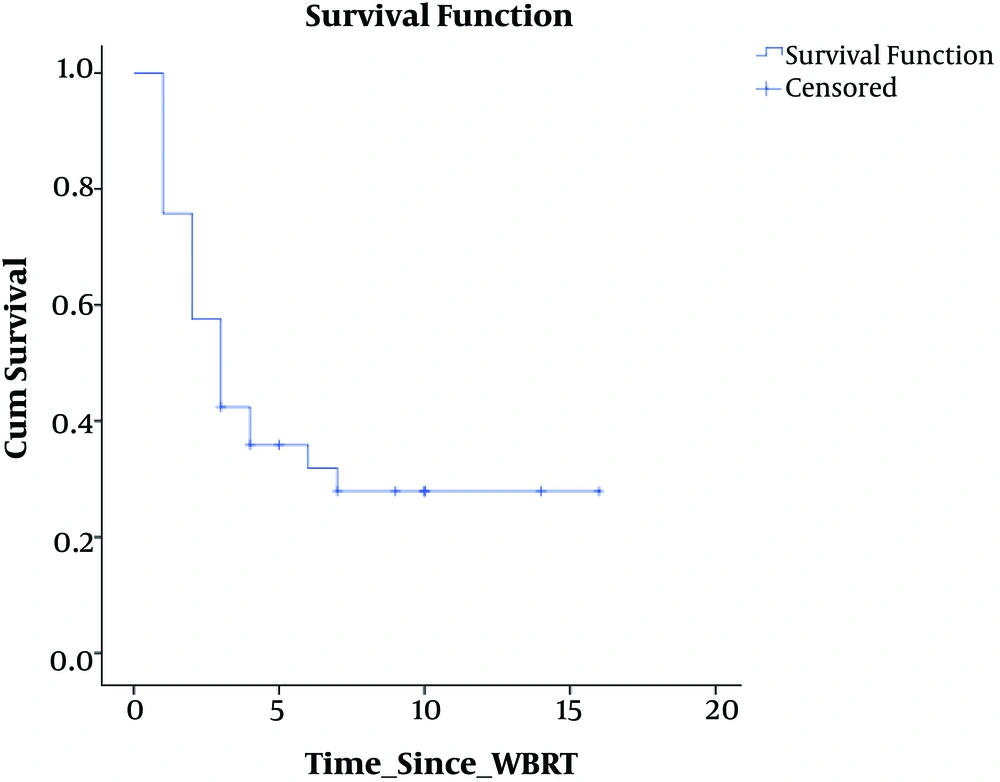
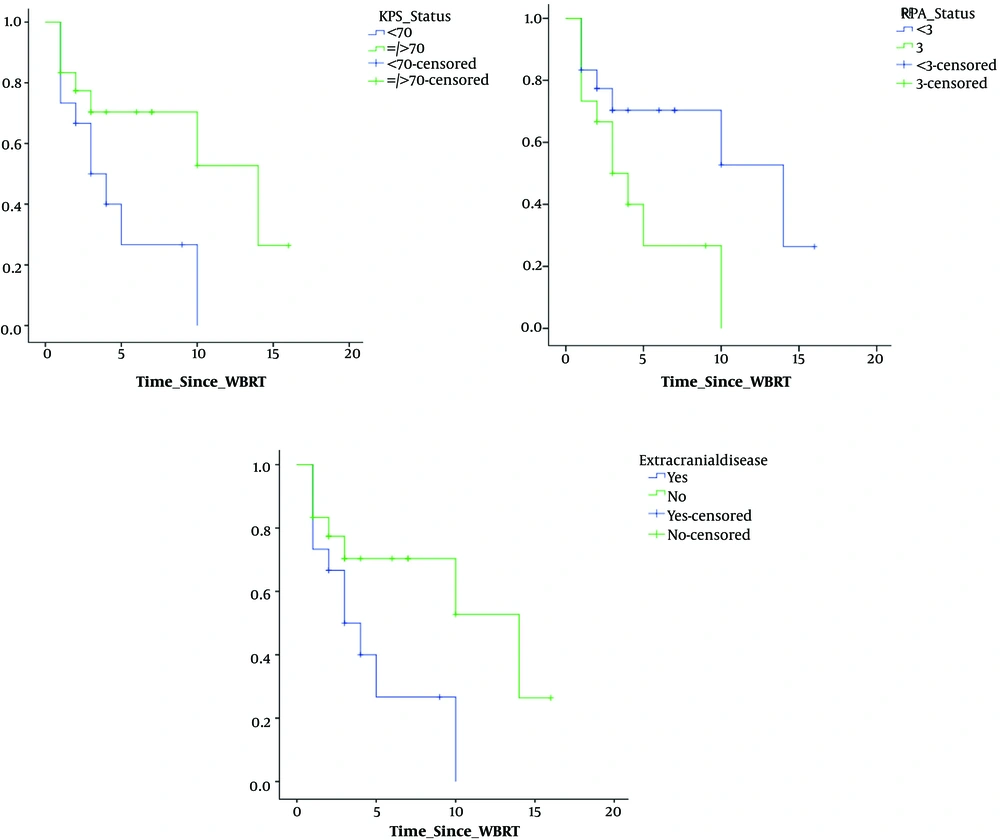
Our cohort had 33 patients, predominantly women (M:F, 12:21) with a median age of 47 years, distributed among all histological primary diagnoses (lung, breast, GI, Gy, GU, MUO: 10, 11, 2, 4, 4, 2) with median KPS of 70 and mean RPA class of 2. Table 1 shows the patient characteristics. Median survival of the cohort was 4 months. At the end of 14 months of follow-up, 30% of the patients were alive with a mean KPS of 70. Figure 2 shows the survival graph of the entire cohort.
| Variables | Findings | ||
|---|---|---|---|
| Age, y, mean ± SD | |||
| 28 - 71 | 46.6 6 ± 11.8 | ||
| Gender, n | |||
| Male | 12 | ||
| Female | 21 | ||
| KPS, mean ± SD | |||
| 50 - 90 | 67.2 ± 13 | ||
| Primary tumor, n | |||
| Lung | 10 | ||
| Breast | 11 | ||
| RCC | 4 | ||
| MUO | 4 | ||
| Gynaec. | 4 | ||
| Cervix | 2 | ||
| Ovary | 2 | ||
| Rectum | 1 | ||
| Esophagus | 1 | ||
| Brain metastasis, n | |||
| 1 | 12 | ||
| 2 - 3 | 7 | ||
| > 3 | 14 | ||
| Location of brain metastasis, n | |||
| Supratentorial | 21 | ||
| Infratentorial | 3 | ||
| Both | 9 | ||
| Extracranial metastasis, n | 15 | ||
| Prior treatment for primary, n | |||
| Yes | 23 | ||
| No | 10 | ||
| Patients diagnosed with brain metastasis at presentation, n | 11 | ||
| Lung | 9 | ||
| MUO | 2 | ||
| Duration between primary treatment and diagnosis of brain metastasis | < 6 months | 6 - 12 months | > 12 months |
| Breast | 4 | 4 | 3 |
| Lung | 9 | 0 | 1 |
| RCC | 1 | 1 | 2 |
| Gynaec. | 3 | 1 | 0 |
| Rectum | - | - | 1 |
| Esophagus | 1 | - | - |
| MRS | Cho/NAA | ||
| Pre-RT | 0.85 | ||
| Post-RT | |||
| 1st month | 0.83 | ||
| 3rd month | 0.85 | ||
| 6th month | 0.74 | ||
| MMSE, mean | |||
| Pre-RT | 25.7 | ||
| Post-RT | |||
| 1st month | 25.6 | ||
| 3rd month | 24.7 | ||
| 6th month | 25 | ||
Patient Character/istics
Repeated measure ANOVA for the trend in the CNI values with time showed that, KPS (P = 0.079), RPA class (P = 0.079), primary diagnosis site (P = 0.049), number of brain metastasis (P = 0.045) showed statistical significance in terms of change in mean value of CNI value at last follow-up.
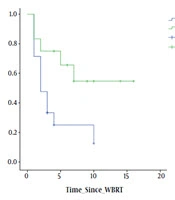
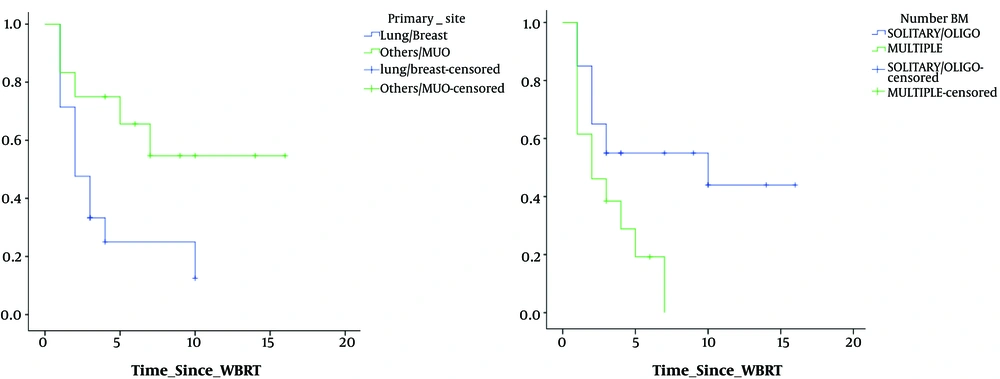
Univariate analysis of various factors for time to deterioration of CNI showed that; site of primary (lung/breast vs. others), P = 0.02 and number of metastasis (solitary/oligo vs. multiple), P = 0.02 showed significance. Figure 3 shows the trends in CNI and correlation with various clinical factors. Univariate analysis of various factors for time to deterioration of MMSE showed that; KPS at presentation (< 70 or > 70), P = 0.04; RPA class (class II or higher), P = 0.04; site of primary (lung/breast vs. others), P = 0.01, presence of extracranial disease (yes or no), P = 0.045, number of metastasis (solitary/oligo vs. multiple), P = 0.06, size of the largest lesion (< 4 cm or > 4 cm), showed (P = 0.02), statistical significance. Figure 4 shows the trends in MMSE and correlation with various clinical factors.
On multivariate analysis, factors with significant correlation to time to deterioration of CNI were duration between last therapy for primary diagnosis and diagnosis of BM (< 6 months or > 6 months, P = 0.012) and presenting complaint of BM (with or without motor deficit/CN palsy, P = 0.023). Factors with significant correlation to time to deterioration of MMSE were age (< 50 or > 50 years, P = 0.089) (this was reaching statistical significance due to small patient number), histology (adenoca vs. other, P = 0.023) and site of BM (supra or infratententorial, P = 0.038).
Factors such as duration of systemic long term chemotherapy prior to WBRT and proximity of the local disease to hippocampus did not show any significance on change in trend of CNI or MMSE values at the last follow-up or either multivariate or univariate analysis.
5. Discussion
Progression of brain metastasis is a much greater cause of neurocognitive dysfunction than WBRT. Compared to best supportive care, WBRT added higher progression free survival and better quality of life (6-8). The WBRT after surgical resection provides higher local and distant control rates without any difference in overall survival (9). WBRT is known to cause neurocognitive decline (10, 11) manifested usually as drop in mini mental state examination score and delayed recall as shown in a randomised controlled study (12). Hippocampus, the seat for conversion and execution of long term to short term memory along with other areas in temporal and basi-frontal lobes are found to be the most common site to develop radiation induced damage (13, 14). Hippocampal avoidance WBRT (HA-WBRT) has been the most widely accepted used method to mitigate the adverse neuro-psychologic effects of WBRT (15-17). It is however essential to understand the basis of patient selection for HA-WBRT (18, 19).
Our study was performed to assess the radiation induced changes in hippocampal and perihippocampal areas after WBRT and mini mental state examination; correlating it with various clinical factors (patient related, systemic disease factors and local disease factors) to identify the patient cohort who may actually benefit from hippocampal avoidance WBRT.
We found that factors such as KPS, RPA, location of primary and number of metastasis had significant impact on variation in the mean value of CNI at the last follow-up. Univariate analysis of various factors on trend of CNI over follow-up shows that patients with lung/breast primary and patients with multiple metastases showed a greater decline in CNI values. Univariate analysis of various factors on trend of MMSE over follow-up shows that patients with lower KPS, higher RPA, lung/breast primary, with extracranial disease, multiple metastasis and size of the largest lesion > 4 cm had a greater decline in MMSE values.
There was negative correlation of change in CNI at follow-up showing that patients older than 50 years, those patients who have received long duration chemotherapy and those patients with presence of BM in the supratentorial location showed a higher decline. There was positive correlation in patients who had longer time duration between the previous therapy and diagnosis of BM and those who did not have motor weakness/CN palsy at presentation of BM. Similarly, on MMSE, positive correlation with patients younger than 50 years, those patients who had non adenocarcinoma histology, infratentorial location of BM showed higher MMSE values at the last follow-up.
Hippocampal avoidance WBRT may be considered in patients with age less than 50 years, KPS of more than 70, RPA class II or higher, non-lung/breast histology, has not received long term systemic chemotherapy, time between last therapy and primary disease is more than 6 months, no motor deficits at presentation of BM, solitary or oligo BM, largest lesion less than 4 cm in size, located atleast 2 cm away from any perihippocampal region and BM located in the infratentorial brain with controlled extracranial disease.
5.1. Conclusions
Our study shows that cognitive functioning after WBRT is influenced by a number of factors including patient related, systemic disease burden and local tumor load. The trend in the decline of CNI index and MMSE scores at each follow-up correlated with various clinical factors and can be used as a guide to aid in selecting patients for hippocampus-avoidance WBRT.