1. Background
Fascioliasis is a zoonotic disease caused by Fasciola hepatica and F. gigantica trematode liver fluke. It is estimated that 17 million people are infected with these parasites, with an additional 91.1 million at risk for this infection (1). In Eastern Europe, South America, and North Africa, F. hepatica is particularly endemic. Turkish studies have reported a prevalence of 0.03% - 0.8% for F. hepatica infection (2). Children are generally more commonly infected than adults, although infections appear to be more serious in women with higher liver or biliary complications (3). Sheep, goat, cattle and other ruminants are mainly contaminated with trematoda. Transmission to people who are unintended final hosts takes place after the ingestion of contaminated aquatic vegetation such as watercress or metacercariae (4, 5). Oviposition occurs within 3 - 4 months after the initial infection, and adult flukes have a life span of up to 10 years. Clinical presentations typically occur after the parasite remains in the liver (6). While two clinical phases of fascioliasis have been recognized in humans, diagnosis and distinguishing between them is often difficult. The acute phase includes larval migration to the liver and lasts 1 - 3 months after metacercariae ingestion. The chronic phase begins when the adult flukes enter the bile ducts, which can last many years. Nearly half of patients during the chronic period may be asymptomatic (7). Many studies have reported cases of mixed-phase fascioliasis in recent years (8-11). Although fasciolosis is generally considered as a notable veterinary problem, human fasciolosis has recently been regarded as the main health issue in numerous countries (12, 13). According to the World Health Organization (WHO) report, Iran has been placed among the six countries recognized to have a serious concern with fasciolosis (2, 14, 15). Fasciolosis has led to two important epidemics in Iran in 2009 and 2011, respectively, which have been the biggest epidemics of fasciolosis in history (16, 17). According to the seroprevalence studies of fasciolosis in Yasouj (2011), Lorestan (2015) and Isfahan (2014), in Iran, anti-Fasciola antibodies were positive in 1.8, 0.7 and 1.7% of the cases, respectively (9, 10, 18).
Several techniques, including serological and parasitological methods are used for the diagnosis of fasciolosis. Parasitological methods have the highest specificity, but some factors such as low rate of parasite eggs, transient infection, and acute and obstructive infections reduce the sensitivity of these methods. Serological tests are usually used for the recognition of anti-Fasciola antibodies in serum samples in the acute phase and ectopic fasciolosis. These methods are appropriate for diagnosing chronic fasciolosis by identifying specific antigens in stool samples and antibodies in the serum as well (19). Therefore, serological methods such as ELISA are commonly used to diagnose human fasciolosis in Iran (20).
2. Objectives
This study aimed to determine the seroprevalence of fascioliasis in Sistan and Baluchestan Province, Southeast of Iran, in 2017.
3. Methods
3.1. The Study Area
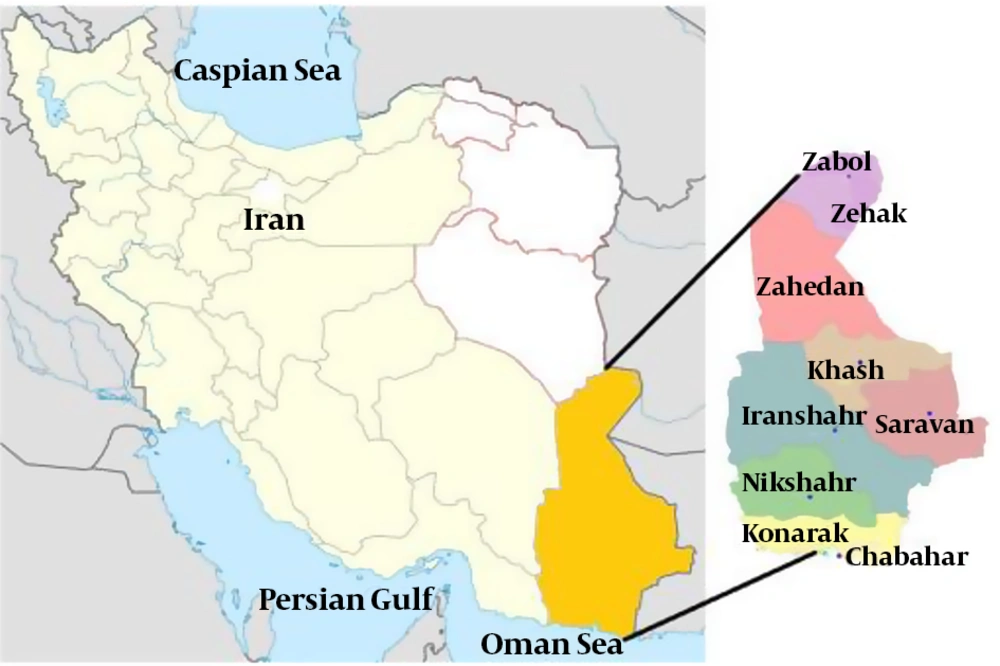
Sistan and Baluchestan Province is located in the Southeast of Iran. The study population consisted of individuals referred to the diagnostic laboratories of Zahedan, Zabol, Chabahar, and Iranshahr. Sampling was performed in the diagnostic laboratories, and 251 human serum samples were collected randomly. Informed consent forms and questionnaires were obtained from the volunteers. The specimens were stored in an ice container during the sampling (Figure 1).
The inclusion criterion was providing consent to participate in this study, and the exclusion criteria were unwillingness to continue participation in the study and having had a positive antibody titer against fasciolosis. Withdrawal of the individuals from the study was one of the limitations of this study. It was also not possible to follow the cases to identify false positives.
3.2. Serum Samples and Enzyme-Linked Immunosorbent Assay (ELISA)
Four milliliters of venous blood specimens were obtained from 251 participants. The blood samples were centrifuged (2500 g) for 10 min to obtain the serum. The serum was separated from clotted blood and was transferred to a -20°C freezer.
In the next step, serum samples were tested using a 96-well ELISA kit (Pishtaz Teb, Iran) specific for Fasciola hepatica. In this technique, microplate wells are coated with a certain amount of Fasciola hepatica-specific antigens.
The samples were diluted 1 to 100 (1/100) with a diluent solution (phosphate buffer solution). Then, 100 µL of diluted specimens and the controls were poured into the wells according to the following procedure: the first well was considered as blank, and the reagent was poured into it. Two wells were selected for the positive control (positive pooled sera containing anti-Fasciola hepatica antibodies diluted in a buffer which contained protein as stabilizer and Kathon CG as preservative), and one well was considered for negative control (negative pooled sera in buffer containing protein as a stabilizer and Kathon CG as preservative). The rest of the wells were used for the samples. After pouring the serum, the wells were covered with a special glue and incubated for 30 minutes at room temperature. Subsequently, the contents of the wells were discarded and washed three times with a prepared wash solution (contained phosphate buffer solution with 0.05% TWEEN 20 as detergent, pH = 6). Afterward, 100 µL of the conjugated solution (contained polyclonal anti-human IgG labeled with HRP) was poured into the wells and covered with glue. The samples were incubated for 30 minutes. The contents of the wells were discarded and washed three times with wash solution. Subsequently,100 µL of dye solution (contained tetramethylbenzidine and hydrogen peroxide) was added to each well, and the samples were incubated for 15 minutes at room temperature and in the dark. The enzymatic reaction was stopped by adding 100 µL of the stopping solution (contained 1 M hydrochloric acid, pH < 1) to each well. To measure the optical absorption of each well, a microplate reader for ELISA (BioTek Instruments) with a 450-nm filter was used, and optical absorption of all the wells was read out against the Blank. After obtaining adsorption, we started the main stages of the sample test. For this purpose, we needed to obtain the F value, which is used to calculate the cut-off point. The cut-off was calculated as 0.25 + X (mean optical absorption of the 9negative controls).
The cut-off was calculated for each microplate. The cut off value of each plate was different, and if the absorbance of a microplate vial was lower than the cut-off point, the result was considered to be negative, and if it was equal or higher, it was considered to be positive.
3.3. Statistical Analysis
Statistical analysis was done using SPSS version 20 (Chicago, IL, USA). A Chi-square test was used for analyzing the data. P value of less than 0.05 was considered significant in all the tests.
4. Results
Using the ELISA method, 251 individuals were sampled; women had the highest infection prevalence (2.70%). The highest rate of infection was in the 20 - 39 years old age group (3.30%), and the lowest rate of infection was observed in those aged 60 - 79 years and over (0%). No significant correlation was observed between the infection and the age of the patients. However, a significant correlation was found between the infection and the use of local freshwater vegetables (P < 0.05; Table 1). The cut-off point was calculated as 0.389 in this study.
| Variable | Number of Samples | Frequency of Anti-Fasciola Antibodies | P Value |
|---|---|---|---|
| Gender | 1.000 | ||
| Male | 28 | 0 (0) | |
| Female | 223 | 6 (2.70) | |
| Age group, y | 0.594 | ||
| 0 - 19 | 50 | 1 (2) | |
| 20 - 39 | 121 | 4 (3.3 0) | |
| 40 - 59 | 67 | 1 (1.50) | |
| 60 - 79 | 12 | 0 (0) | |
| > 80 | 1 | 0 (0) | |
| Location of residence | 0.523 | ||
| Zahedan | 231 | 6 (2.60) | |
| Zabol | 15 | 0 (0) | |
| Chabahar | 2 | 0 (0) | |
| Iranshahr | 3 | 0 (0) | |
| Use of local freshwater vegetables | 0.012 | ||
| Yes | 121 | 6 (2.95) | |
| No | 130 | 0 (0) |
Seroprevalence of Anti-Fasciola Antibodies According to the Epidemiological Factorsa
5. Discussion
In recent years, cases of fascioliasis in many countries and a shift in disease outlook have been reported steadily (21-23). Studies of fascioliasis in endemic areas around the world have reported a very low prevalence (< 1%) in Basse Normandie and Corsica in France, moderate prevalence (1% - 10%) in Porto in Portugal, Alexandria, Nile Delta, and Sharkia in Egypt, while Corazal in Puerto Rico and Cajamarca in Peru have been found to have a high prevalence (> 10%). In three studies performed in Van Province, F. hepatica eggs were detected in 5 (2.4%) of the 206 people in the town of Ercis, in 2 (0.68%) of the 293 students in the 7 - 15 age group, and in 1 (0.03%) of the 3,534 people aged 14 years and above in Van City (11, 22-25).
Sarkari et al. (18) reported a 1.8% prevalence of fascioliasis in Yasuj, Guilan, and Northern Iran. On the other hand, a small outbreak was reported in Kermanshah, Western Iran (10, 18, 26). Espinoza et al. (9) reported the prevalence rate of fascioliasis to be 1.7% in urban and rural areas.
Of course, the positive cases of fascioliasis in this study were higher than those of similar studies, and this may have been due to the use of low sensitivity and specificity commercial kits. In the present study, the total prevalence of human fascioliasis infection was estimated to be 2.40% in the province of Sistan and Baluchestan. We found statistically significant differences between the two sexes regarding infection rates. Findings showed that the rate of infection was higher among women. No significant correlation was observed between the infection prevalence and age of the patients (P > 0.05).
The highest infection rate was found in the 20 - 39 years old age group (3.30%), and the lowest rate of infection was reported in those aged 60 - 79 years old and above (0%). The higher rate of fascioliasis in females than in males can be attributed to the fact that women consume green aquatic plants more often than men do. The higher rate of infection in people aged 20 - 39 years may reflect the increased possibility of encountering the parasite with aging. The higher infection rates among the 20 - 39 years old age group in the present study compared to other studies maybe due to the fact that in this region (Sistan and Baluchestan Province), people in this age group are more likely to be in contact with the contaminated environment.
5.1. Conclusions
The results of the present study indicated that fascioliasis is native to the Zahedan Region. Regarding the hygienic issues and health implications, as well as the economic impacts, the burden of this infection in the region is significant; therefore, it is recommended that the following issues be taken into consideration by the researchers and executive authorities of the region: (1) to conduct more extensive studies in the area using serologic tests such as ELISA and to use ultrasound screening; (2) to investigate the possible infection of the intermediate and final hosts in the region; (3) to conduct interventional studies in order to control the spread of the disease in the area; (4) to review and estimate the burden of the disease imposed on the people of the region; (5) to educate the people of the region about the ways the infection spreads and to increase their understanding of the disease and its prevention methods and to train them on how to wash vegetables and how to clean a slaughterhouse; and (6) to help the patients with their treatment and grant financial support and to expand social security for the people of this region.