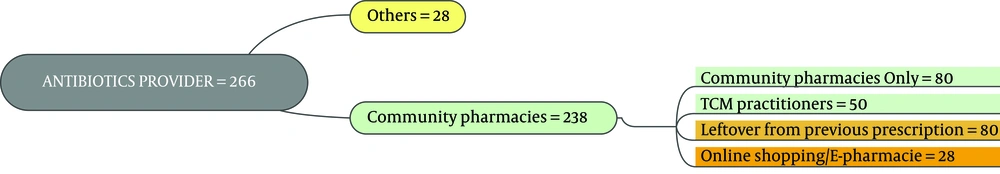1. Background
Globally, antibiotics are one of the most commonly used medicines, specifically in developing countries where infectious diseases are still the most common cause of death (1). Self-medication refers to any attempt for self-treatment after self-diagnosis of the health issue or medical problem by taking medicines without any professional advice or authorized prescriptions (2). This type of medical solution is pervasive in most parts of the world, and 50% of all used antibiotics are purchased without any prescription (3). Antibiotic self-medication may lead to health hazards in developing countries that face poverty, inaccessibility, lack of medical professionals, poor quality of health equipment, irregular distribution of medicines, and decreasing patients’ trust in physicians (4). Considering the rapid growth of microbial resistance to antibiotics (5-7), the World Health Organization (WHO) has begun implementing a global program entitled Microbial Resistance to Microorganisms in 2001 (8). Despite these WHO activities, the use of antibiotics without prescriptions is increasing in developing countries (9).
Taking an inappropriate antibiotic and improper antibiotic usage may cause (1) microbial resistance, (2) spreading of some diseases such as pseudo-membranous colitis, (3) economically negative impact (8). Researchers previously found that self-treatment with antibiotics had a significant relationship with age, income, education, and patient satisfaction of former effective usage (10, 11). It can be the result of patients’ experience of the effectiveness of previous treatment (10). Increasing public knowledge of antibiotic treatment plays an important role in decreasing antibiotic resistance (12, 13). General awareness of antibiotic resistance in countries with high antibiotic resistance is not reasonable.
2. Objectives
considering the current situation of antibiotic resistance and demographic characteristics of visitors of a medical center located in the Northwest of Tehran, this research was conducted to investigate antibiotic self-treatment in the community.
3. Methods
This study was conducted to investigate antibiotic self-treatment in the community, initially considering the current situation of antibiotic resistance and the patients’ demographic characteristics of a medical center in the Northwest of Tehran. Three questionnaires were designed based on demographic characteristics of gender, occupation, education, marital status and health insurance (five questions), general knowledge about antibiotics (five questions), and the experience of antibiotics self-medication (24 questions). Between questionnaires 2 and 3, there is a filtering question of whether or not the participant has the experience of antibiotic self-medication. Based on the response to this question, the participants were categorized into two groups. If the answer was negative, so there was no need to answer the rest of the questions around the antibiotics self-medication in questionnaire 3. Otherwise, they have been asked to complete the questionnaire 3 that included 24 multiple-choice questions. To respond to some questions, the participants have the option to select more than one choice, such as experienced side effects or the type of used antibiotics. In this study, the participants who had experiences of antibiotic self-medication were called group two. All participants were informed of the method, background, target, implementation, information confidentiality, and releasing results conditions and completed written informed consent form before the study.
3.1. Statistical Analysis
Categorical variables were expressed as frequencies, whereas numerical variables (age) were presented as means and standard deviation (SD) as data were normally distributed. We used the chi-square test (Fisher’s exact) for comparing the study variables such as gender, education level, marital status, insurance status, and occupation. Based on collected data, at least 1,024 records were required to make a predictable model, and 434 records were not sufficient for this purpose. All statistical analyses were performed using STATA software (version 22), and only p-values less than 0.05 were considered significant.
4. Results
The average age of participants was between 23 and 49, and most of them were married and high-educated. The participants were asked what the main side effect or adverse reaction of antibiotics was. The first group without antibiotic self-medication experience declared vaginal thrush and antibiotic resistance as antibiotic side effects. The second group who experienced antibiotic self-medication pointed to nausea and vomiting as antibiotics side effects.
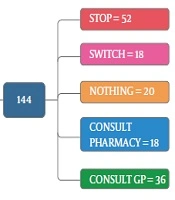
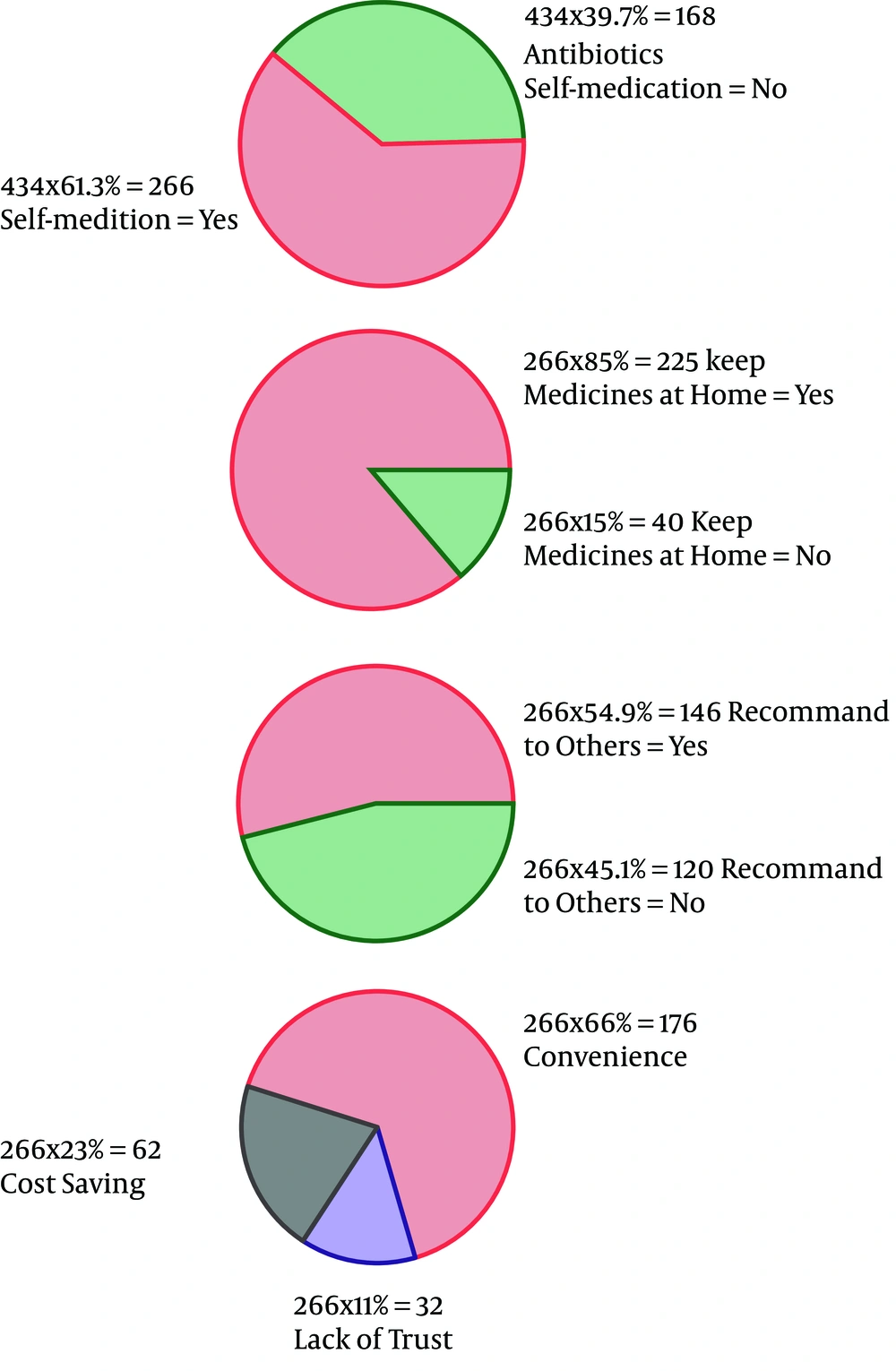
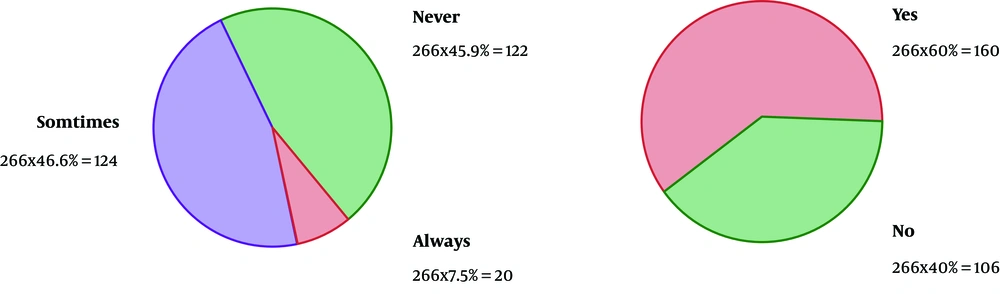
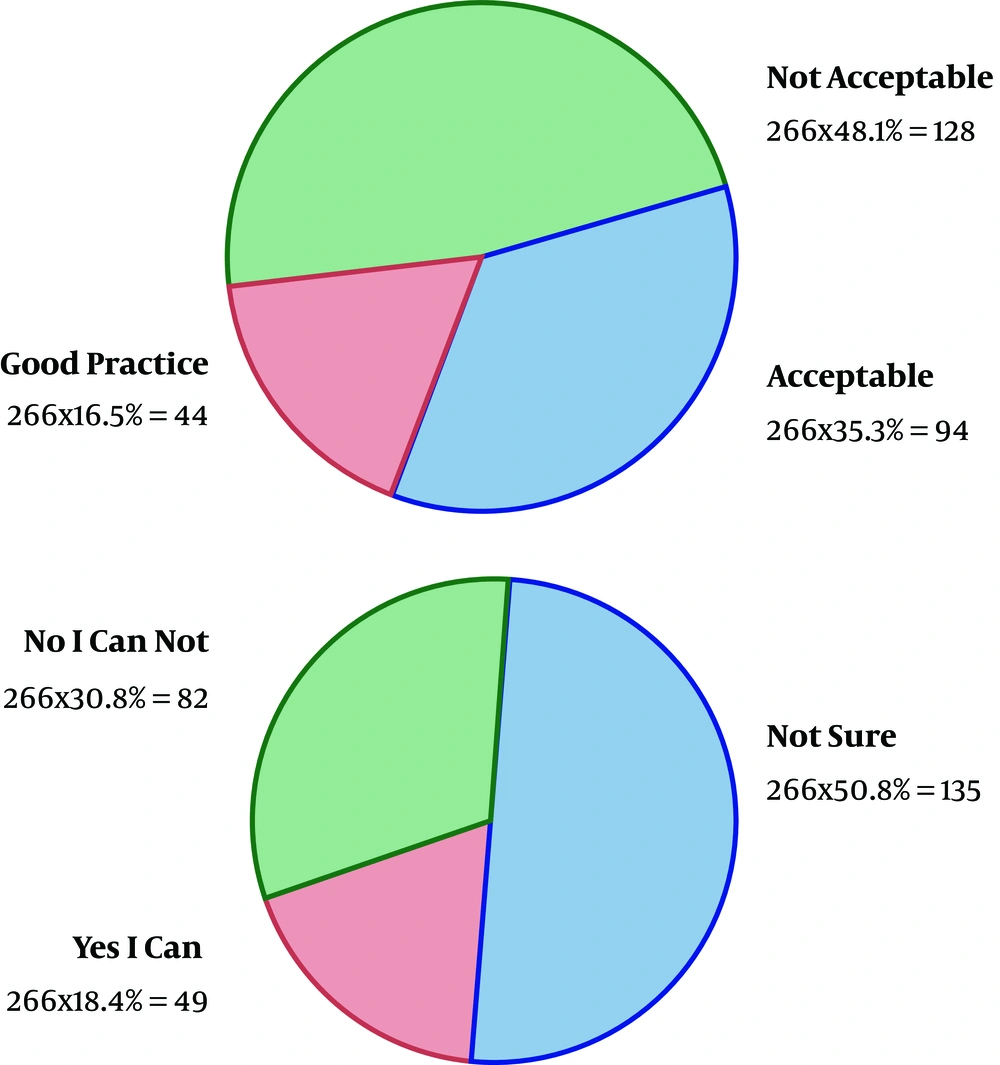
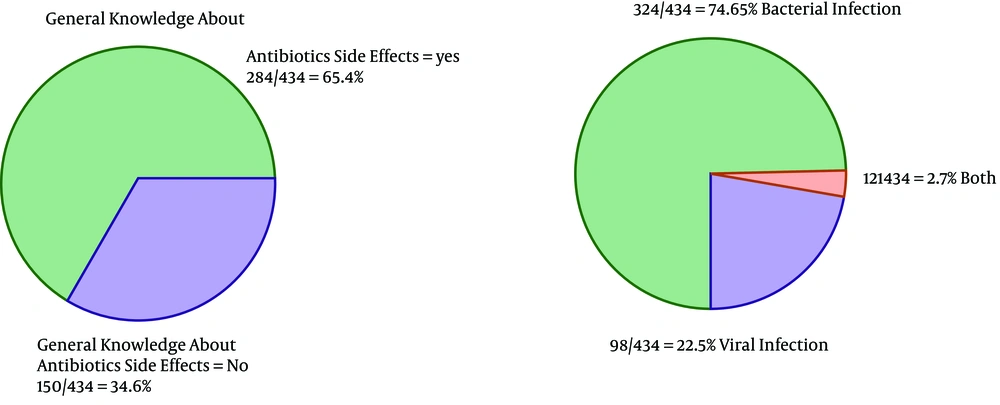
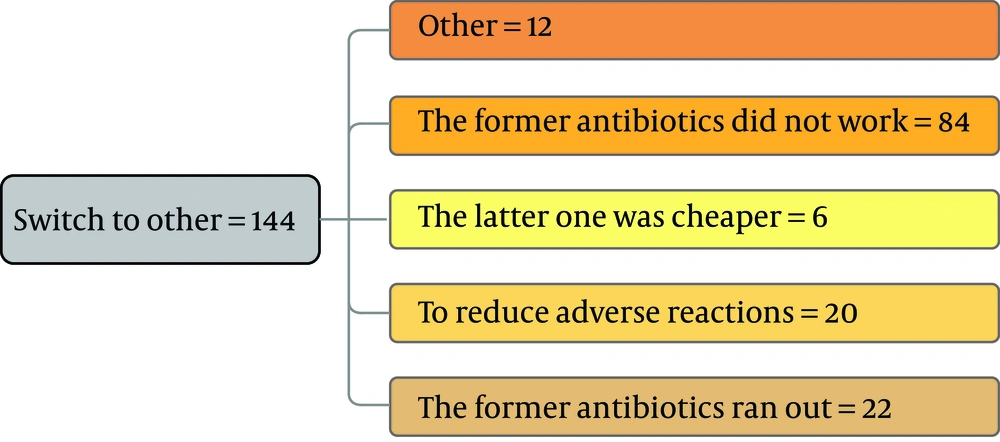
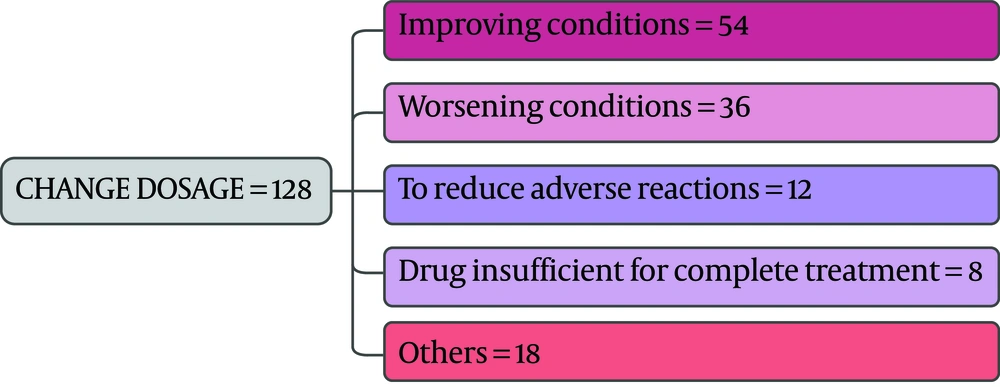
As the first row of Figure 1 illustrates, 61.3% of the participants had the experience of antibiotics self-medication (group 2). Moreover, 0.85% of the group 2 kept some medicines at home (second row), and 54.9 % of them recommend the used antibiotic to others (third row). The reason for antibiotic self-medication for 66.2%of them was convenience, for 23.3% of them were cost-saving, and for 11.4% was the lack of trust in physicians (last row). As Figure 2 illustrates, 46.6% sometimes switched antibiotics, and their reason was lack of efficiency. 45.9% never switched antibiotics (left) 60.2% had the experience of taking the same medicines with different names at the same time (right). When the participants were asked about the efficiency of antibiotic self-medication, 48.1% believed that it was inefficient, 16.5% were not sure, while 35.3% found it efficient (Figure 3). Figure 4 on the left side shows that 65% know about side effects, whereas the right side indicates that 75% believe antibiotics affect bacterial infections. Figure 5 illustrates 144 out of 266 participants of the group two switched to other antibiotics, it also shows their reasons. Figure 6 illustrates 128 out of 266 participants of the group two changed the dosage of antibiotics, it also shows their reasons. Correspondingly, 56.6% never changed the dosage of antibiotics deliberately, while 36.9% occasionally changed the dosage. Condition improvement was their reason for changing the dosage. Figure 7 illustrates the antibiotic providers for 266 participants of the group two. Accordingly, 83.5% usually provided antibiotics from community pharmacies, while 23.8% used leftover medicine.
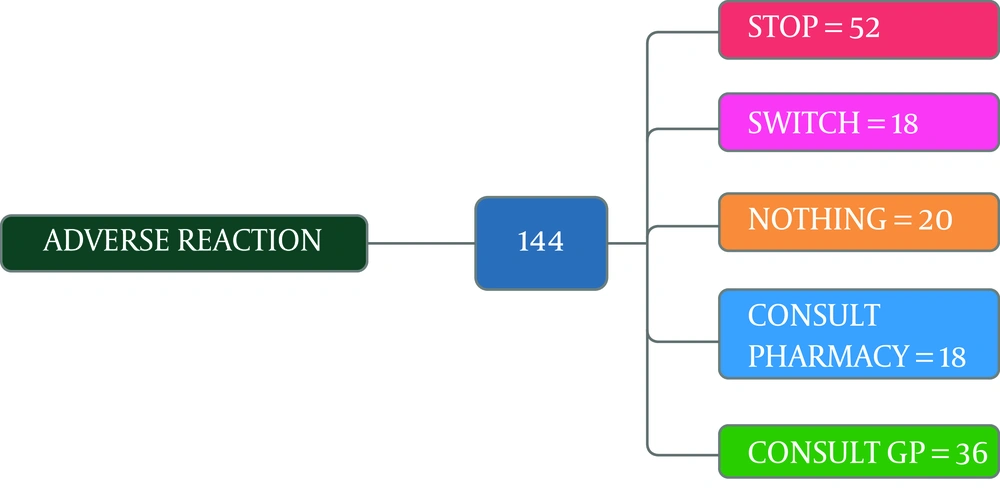
Figure 8 illustrates 144 out of 266 participants of the group two had adverse reaction experiences and what they did after these experiences. Most participants stopped taking medicines after an adverse reaction. In this study, 71.4% of self-medicated participants (group 2) used antibiotics to treat sore throat, 51.2% of them in the self-medication group chose the medicines for treatment based on the last prescribed medicine, and 45.6% relied on their own former experience. In selecting antibiotics, 53.4% considered the type of medicines, 56.6 % occasionally checked the instructions on the medicine package, 30.3% always checked this instruction, 53% of the group two participants fully understood the instructions, 35.9% partially understood, and 11.1% did not understand at all. Also, 46.6% stopped taking antibiotics when their illness symptoms disappeared (19.5%). Amoxicillin, Co-amoxiclav, and Cefixime were the most common antibiotics used, respectively. Table 1 summarizes the comparison of different five factors, including gender, occupation, education, marital status, and health insurance cover in the self-medication and non-self-medication groups. These results were not significant at P < 0.05.
| Variable | Non Self-Medication, No. (%) | Self-Medication, No. (%) | Total | Chi-Square | P Value |
|---|---|---|---|---|---|
| Gender | 1.21 | 0.027 | |||
| Male | 122 (116.45) [0.26] | 68 (73.55) [.42] | 190 | ||
| Female | 144 (149.55) [0.21] | 100 (94.45) [0.33] | 244 | ||
| Occupation | 0.12 | 0.72 | |||
| Medical | 100 (101.74) [0.03] | 66 (64.26) [0.05] | 166 | ||
| Non-medical | 166 (164.26) [0.02] | 102 (103.74) [0.03] | 268 | ||
| Health insurance | 0.107 | 0.74 | |||
| Yes | 234 (232.9) [0.01] | 146 (147.1) [0.01] | 380 | ||
| No | 32 (33.1) [0.04] | 22 (20.9) [0.06] | 54 | ||
| Marital status | 3.31 | 0.19 | |||
| Married | 148 (142.85) [0.19] | 84 (89.15) [0.30] | 232 | ||
| Widow | 14 (18.47) [1.08] | 16 (11.53) [1.74] | 30 | ||
| Single | 104 (104.68) [0.00] | 66 (65.32) [0.01] | 170 | ||
| Education | 5.64 | 0.059 | |||
| Undergrad | 10 (9.81) [0.00] | 6 (6.19) [0.01] | 16 | ||
| Graduated | 50 (60.06) [1.69] | 48 (37.94) [2.67] | 98 | ||
| Post-grad | 206 (196.13) [0.50] | 114 (123.87) [0.79] | 320 |
5. Discussion
Most researchers focused on patients’ views and their opinions regarding antibiotic self-medication (14-17). Healthcare professionals and medical advisers, particularly pharmacists, play a significant role in modifying these beliefs and habits (18). The frequency of self-medication in this survey (61.3%) was more than the mean estimation of antimicrobial self-medication in the countries with low and middle-range income (38.8%) (15).
5.1. Prevalence Variation
The results of recent research indicate that the prevalence of self-medication varies between 7.3% to 85.59%, with an overall prevalence of 42.64% (19).
5.2. Reasons for Prevalence Variation
The reasons for this prevalence variation are differences in social determinants of health, tradition, culture, economic, developmental status, socioeconomic culture, personal traits, and the healthcare system (20, 21). On the other hand, methodology, study prevalence variation, sample population, and recall time may cause this variation. The other series of parameters for the prevalence of self-medication include the short distance between pharmacies and residential area or long distance to the healthcare facility, negligence, minor illness, inappropriate attitude of health workers (impolite, corrupt, unclean), re-treatment of similar disease, and lack of health personnel (19).
5.3. Variation
Research results of Aziz et al. (2018) confirmed the researches findings in developing countries (18). Based on these researches, the prevalence of antibiotic self-medication is 17% in Pavyde et al. (15), 52% in Kandelaki et al. (19), 4.4% in Widayati et al. (20), and 3.4% in Gilani et al. studies (21).
5.4. Age
In our study, antibiotic self-medication had no significant relationship with age. In the study of Al-Azzam et al. in Jordan, the highest frequency of antibiotic self-medication was at 18 to 39 years. Study results of Bulario et al. (2018) indicated that age was the only factor significantly associated with antibiotic self-medication (22, 23).
5.5. Children
Based on studies of Togoobaatar et al. and Alele, treating a child with a non-prescribed antibiotic is increased with the child’s age (24, 25). The reason underlying this increase is mothers’ cautiousness and fears to take medicines for infants or early-stage children. Alele (25) described since mothers are cautious, or have various fears to take medicines for infants or early-stage children, so antibiotic self-medication increases with the child’s age.
5.6. Knowledge
According to reports of studies, mothers without jobs and mothers with higher education without any health insurance cover more than mothers who employed attempt to treat their children by antibiotic self-medication. Based on Lithuania’s research, there is no relation between knowledge about antibiotics and self-medication (16). On the other hand, free study results in the USA indicate that the lack of sufficient knowledge is one of the reasons behind antibiotics self-medication (26).
5.7. Gender
In our study, the rate of antibiotic self-medication in the male population is 64.2%, and in the women population, is 59.1%. These results were confirmed by Grigoryan et al. (27) and Widayati et al. (28). They established that antibiotic self-medication in men population is higher than the women population.
5.8. Cause
The most common cause of self-medication in our study is the ease of access to these medicines. Whereas, Horumpende et al. confirmed that the lack of knowledge, the urgent need to treat illness, and the expense of access to health requirements lead to self-using of antibiotics (29). In the Ershadpour survey, most people believe that there is no risk for antibiotic self-medication (30). Some of the other reasons for antibiotic self-medication in Iran are patients’ lack of trust in the quality of medicine, preferring the imported brand and the availability of over the counterantibiotics (29). The result of our study showed that 46.6% of the participants stopped taking antibiotics when their symptoms had disappeared. Many researchers studied to find out why people stop taking medicines after their symptoms disappeared. Their results indicate that people have not sufficient knowledge about improper treatment taking antibiotics and adverse reactions caused by it (31).
5.9. Affect
In the current study, 73% of participants in the group two believe that antibiotics can affect bacterial infections. Ekambi et al. and Bulario et al. (2018) have reported that in the opinion of 74.7 % of their survey participants, antibiotics have affected bacterial infection (20, 23). Generally, most people do not have accurate and comprehensive information regarding antibiotics, their properties, side effect, and fighting against microbes and infections (20). A community-based study in developing countries has reported that most adult people believe that bacteria and viruses are the same, and antibiotics can kill both of them (32).
5.10. Type
The most commonly used antibiotic in our study was amoxicillin, which is consistent with the results of the Nepal (1), whereas, in the study of Ajibola et al., the most common antibiotic was ampicillin (33).
5.11. Health Issue
In our study, most people took antibiotics to treat sore throat, and the most common side effect was nausea. According to the study by Bulario et al., most participants took antibiotics for fever, cough, and cold (23). According to Horumpende et al. research, in Barangay Tandang Sora, Quezon City cough, throat irritation, fever, and gastrointestinal tract diseases such as diarrhea and vomiting mostly make people take antibiotics without physician advice (29). Based on Gebeyehu study, health issues, which lead people to take antibiotics without prescription are tract symptoms (74.6%), diarrhea (74.4%), and physical injury/wound (64.3%), respectively (31).
In fact, self-medication has some advantages for healthcare systems in some cases. It may reduce pressure on medical services, leading to optimal utilization of facilities and clinical skills. These clinical skills are associated with general practitioners and specialist pharmacists, paramedics, and those who prescribe medicines. Antibiotic resistance is a modern medical challenge (15, 17, 34); thus, the prevalence of antibiotics self-medication needs to be controlled. Nowadays, due to the access to all available information on the internet, some patients tend to use self-medications. The lack of knowledge of some patients about some medicines, the treatment period, and its side effects may increase the risk of self-medication. Totally, 61.3% of the participants of the present study had the experience of self-medication with antibiotics. It means almost a large number of people prescribe and consume antibiotics for themselves without authorized prescriptions and they do not need it. Although most of them evaluate that the final treatment result was not successful, the lack of knowledge of some patients about some medicines, the treatment period, and its side effects may increase the risk of self-medication (20, 35).
5.12. Conclusion
Antibiotic self-medication can cause some severe adverse reactions. Obtained results from participants’ responses highlighted the role of local community pharmacy as an available and reliable health advisor and medicine supplier. In conclusion, pharmacies can support community health by providing medicine leaflets. These leaflets include information about the proper dosage of medicine, instruction, the treatment period, side effects, and adverse reactions.