1. Background
Osteoporosis, osteoarthritis, and sarcopenia are the most common musculoskeletal disorders (1). Osteoarthritis is a chronic degenerative joint disease characterized by pain, articular stiffness, and an incremental decrease in the articular function, which significantly affects the quality of life (2). Synovial membrane inflammation, articular capsule thickening, muscle weakness, and new bone formation are complications of osteoarthritis. Therefore, osteoarthritis affects not only the tissues inside the capsule but also the tissues surrounding the joint, including the muscle (3). Due to its nature, it is reported that osteoarthritis is closely associated with sarcopenia (4, 5).
Muscle damage and weakness in people with osteoarthritis are usually associated with muscle fiber atrophy (6). It has been reported that in subjects with hip and knee articular osteoarthritis, the cross-sectional area of the muscle reduces by 12 to 19% (7).
Various methods are suggested to inhibit the negative effects of osteoarthritis. One of the most common of these methods is the use of physical training, which includes resistance, stretching, or aerobic training in water and land. Both high and low-intensity aerobic training are reported to be suitable for people with osteoarthritis (8). Since the main goal of osteoarthritis treatment is to alleviate joint pain, minimize motor disability, and improve aerobic fitness, therefore, aerobic or resistance training can be recommended as the main treatment for people with mild to moderate osteoarthritis (9).
There is evidence that the use of stem cells can have therapeutic effects on osteoarthritis (10). In animal models, stem cell therapy can protect or improve induced osteoarthritis (11). Stem cells do not appear to become chondrocytes, but rather suppress synovial activation and indirectly reduce cartilage damage (12).
Hyaluronic acid has also recently been used as a very effective treatment for osteoarthritis pain (13). Cell proliferation is one of the most important stages of muscle regeneration after injury (14). Hyaluronic acid is essential for the removal of fibroblasts from the extracellular matrix and mitosis (15).
2. Objectives
The current study aimed to investigate the independent and combined effect of aerobic training, stem cells, and hyaluronic acid treatments on the quadriceps muscle fiber count in the rat model of knee osteoarthritis.
3. Methods
3.1. Ethical Considerations
The present study was in accordance with the National Institute of Health Publication, and all the ethical principles were observed regarding working with laboratory animals. It was also approved by the Animal Care and Maintenance Committee of the Islamic Azad University of Sari Branch (no.: IR.MIAU.RECE.1396.132).
3.2. Animals
Fifty four Wistar male rats, aging 2 to 3 months with a mean initial weight of 250 to 300 g, were divided into 9 groups (each with six subjects), including healthy-control, osteoarthritis- control, osteoarthritis-training, osteoarthritis-stem cell, osteoarthritis-hyaluronic acid, osteoarthritis-hyaluronic-stem cell, osteoarthritis-stem cell-training, osteoarthritis-hyaluronic acid-training, and osteoarthritis-stem cell-hyaluronic acid-training. Rats were housed in cages (each shelf held three rats) with dimensions of 42 × 26.5 × 15 and in a room with controlled conditions (i.e. 22 ± 2°C, 55 ± 5 % humidity, and a 12:12 light-dark cycle with appropriate ventilation). The animals were fed a daily pellet of 10 g per 100 g of body weight made by Karaj Behparvar Co. (according to weekly weighing) and had access to free water through special bottles.
3.3. Induction of Knee Osteoarthritis
Osteoarthritis was induced using a surgical procedure developed by Bendele et al. (16). Rats were anesthetized by ketamine (30 - 50 mg/kg) and xylazine (3 - 5 mg/kg). After correcting the right knee, a 1 cm incision was made to make the knee joint appear. Then, the knee joint was immediately opened by a lateral dislocation of the patellar bone and the patellar ligament. A longitudinal incision was made through the parapatellar medial incision. The lateral dislocation of the patella and patellar ligament was performed by forceps and then an incomplete incision was made in the anterior cruciate ligament without damage to the articular cartilage and other ligaments. Finally, an articular capsule with 6 absorbable sutures and skin with 6 silk sutures were closed. The rats were fed standard food and water for three weeks (16).
3.4. Hyaluronic Acid Treatment
In the hyaluronic acid receiving groups, hyaluronic acid was injected into the injured joint in three separate weeks and once a week. The intra-articular injections of 25 µL of HA (Hyalgan® sodium hyaluronate; FidiaFarmaceutici S.p.A., AbanoTerme, Italy) was administered using a 27 gauge, 0.5-inch needle. The dose calculated for rats was determined with reference to the human, as suggested by Paget and Barnes (17).
3.5. MSCs Intra-Articular Preparation and Injection
MSCs were extracted from the bone marrow of healthy Wistar rats (250 - 300 g) after inducing anesthesia with ketamine (30 - 50 mg/kg) and xylazine (3 - 5 mg/kg). Isolated MSCs were cultured in the DMEM medium with 20% FBS incubated overnight to select adherent cells. Cultures were changed from flask medium every three days to remove unadhered cells, and MSCs reached < 90% purity after 3 to 4 times passage and were selected for injection. Rats in the MSCs group received 1 × 106 cells/kg by intra-articular injection of MSCs. MSCs were injected into the right knee joint of the rats (18).
3.6. Preparation of Tissue Samples and the Way to Measure the Number of Muscle Fibers
After performing the study, all animals were anesthetized and sacrificed by an intraperitoneal injection of ketamine (60 mg/kg of body weight) and xylazine (5 mg/kg of body weight), followed by 12 to 14 hours of fasting and 48 hours after the last training session and injections (to eliminate the acute effects of training and supplementation), a completely similar process was used for all cases. The cartilage and quadriceps muscle were carefully separated and washed with distilled water in 10% formalin (Merck, Germany). After 48 hours of tissue fixation, tissue samples underwent paraffin molding for histological preparation. The specimens were then placed in ascending series of alcohols from 70% to 100% (Merck, Germany), and then they were exposed to xylol (Merck, Germany) and paraffin (Merck, Germany) and molded; then using a microtome (Leica, USA), a 5-micron incision was provided. Each specimen was then stained with hematoxylin and eosin (Merck, Germany) and imaged by light microscopy (Lambda, USA). From each sample, 5 different fields were randomly evaluated at 400 magnification using Image J software (NIH USA), and the percentage of osteoarthritis injury and the number of peripheral muscle nuclei were counted by software.
3.7. Training Protocol
One month after surgery, one week was spent for familiarizing and adjusting to the research environment and the treadmill. For this purpose, animals were planned to run on the treadmill 3 days a week for 10 minutes at a speed of 16 m/min with 60% - 70% of Vo2max and 0% slope. No stimuli were applied to move the animals at this stage. The training protocol consisted of 30 minutes of running on the treadmill with no slope at a speed of 16 m/min for the first week. Then, to observe the principle of overload, the training duration progressively reached 50 minutes in the eighth week. Before each training session began and after it was completed, the animals performed warm-up and cooling for five minutes at a speed of 8 m/min. The control group was only on the off treadmill during the training session with the same time period.
3.8. Statistical Analyses
Descriptive statistics were used to classify and determine the distribution parameters. The Shapiro-Wilk test was used to detect data distribution. To investigate the changes between the groups, a three-way analysis of variance (ANOVA) and t-test were used. The results were followed with a Bonferroni post hoc test. All significant levels were considered as P < 0.05 (using the SPSS (version 22)) for determining the difference between variables.
4. Results
4.1. Articular Cartilage Area Repair in Different Experimental Groups
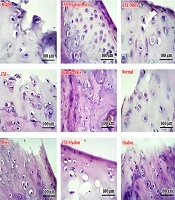
According to the results of the histology section of the articular cartilage, the extent of tissue damage in the articular cartilage in the groups receiving hyaluronic acid alone or in the bone marrow stem cells was not significantly different from the observed model in articular cartilage. Although new cells were found at this site, they were not able to lead to complete repair of the damaged area. On the other hand, the results of the combination therapy section that was associated with exercise training indicated significant improvement. Based on the results shown in the tissue images, exercise training along with stem cell therapy or hyaluronic acid also produced a 50% improvement in tissue, but the combination of all factors led to a better chondrocyte orientation (Figure 1).
4.2. Evaluation of the Quadriceps Muscle Fiber Count in Different Experimental Groups
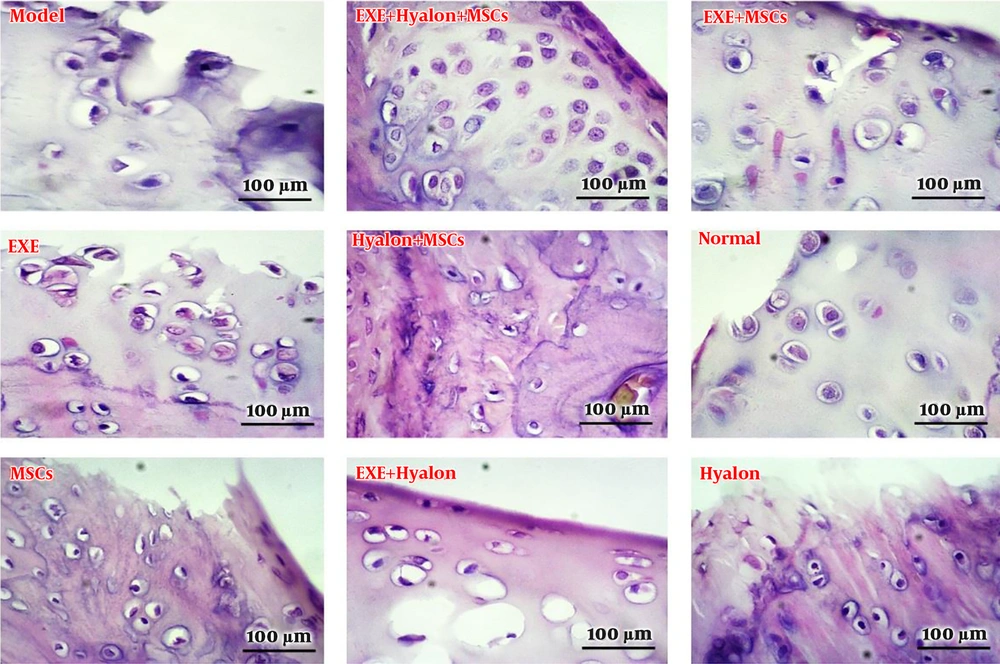
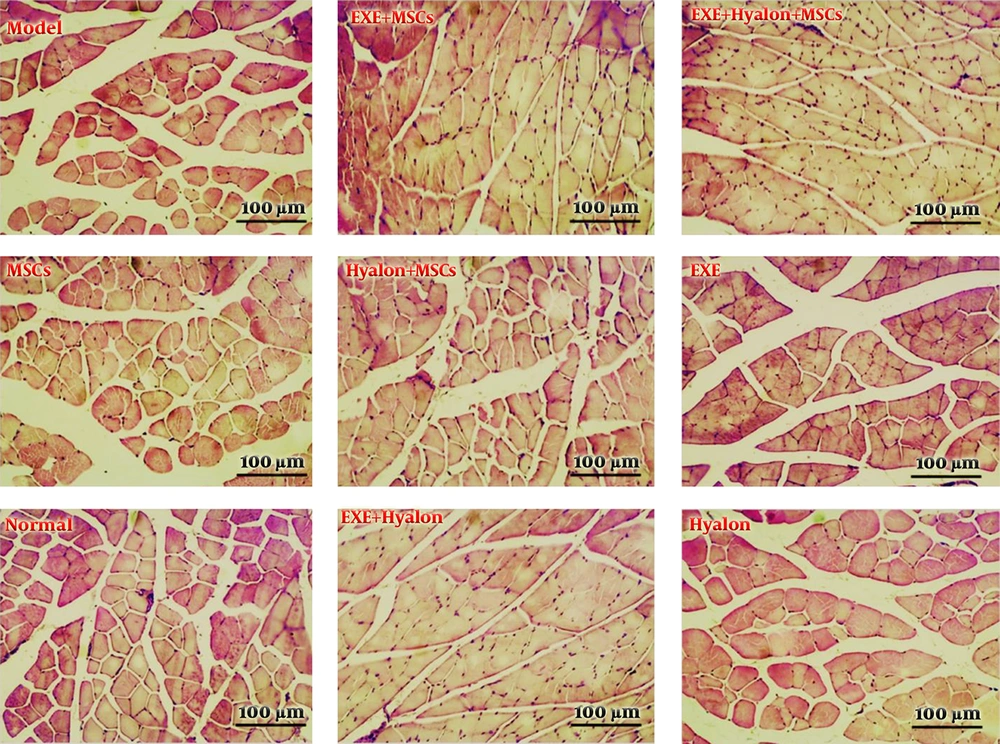
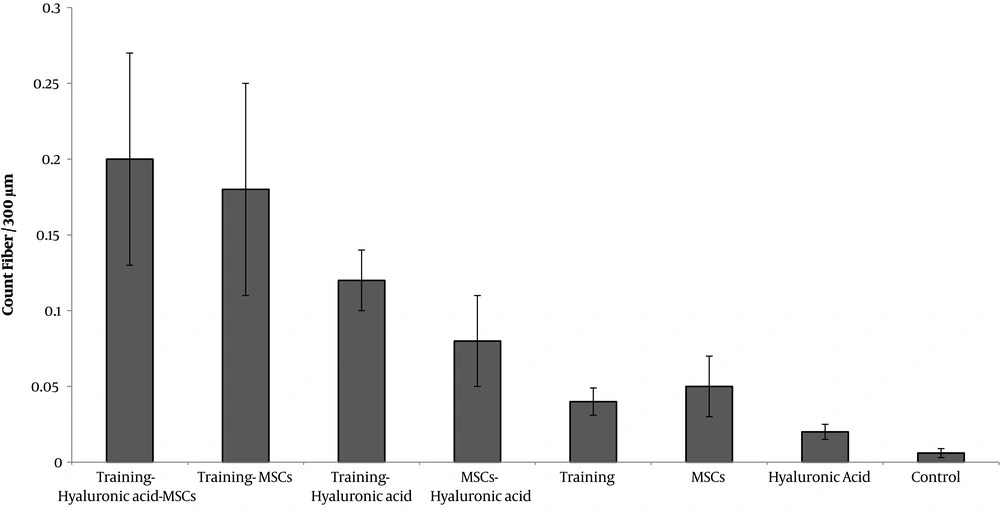
Based on the results in this section, the number of muscle fibers with a magnification of 400 and at a cross-section of 300 µm was significantly reduced in the model group; besides, according to the results, the quadriceps muscle fiber count in the combination groups was significantly higher than the model group or the treatment group alone (Figures 2-4).
Histological cross-sections of quadriceps muscle fiber count in different groups examined. Each cell nucleus is considered to represent muscle fiber. Cross-sections were prepared at a magnification of 400× and stained with hematoxylin and eosin. EXE: Aerobic exercise training; Hyalon, Hyaluronic acid; MSCs, stem cell therapy.
4.3. The Effect of Study Interventions on Quadriceps Muscle Fiber Count
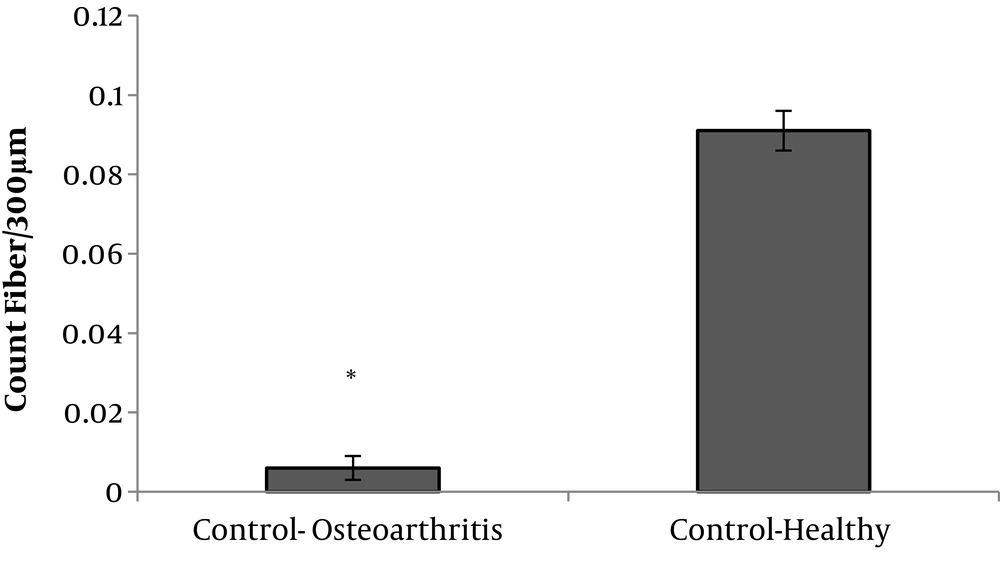
The results showed that the fiber count significantly decreased responses to knee osteoarthritis (P = 0.001) (Figure 3).
Aerobic exercise training significantly increased the fiber count (F = 3006.846, P = 0.001, µ = 0.987). Stem cell therapy also increased the fiber count significantly (F = 2492.665, P = 0.001, µ = 0.984). Hyaluronic acid intake significantly increased the fiber count (F = 794.089, P = 0.001, µ = 0.952).
Interaction of aerobic exercise training and stem cell therapy (F = 39.148, P = 0.001, µ = 0.495) increased the fiber count. A combination of aerobic exercise training and stem cell therapy increased the fiber count more compared to each one alone.
Although the number of fibers in the aerobic exercise training and hyaluronic acid combination group was higher than that of the hyaluronic acid alone group, their combination (F = 0.734, P = 0.397, µ = 0.018) had no significant interactive effect on the fiber count. Interaction of stem cell therapy and hyaluronic acid (F = 0.164, P = 0.397, µ = 0.004) also had no additive effect on the fiber count. On the other hand, when the three interventions of the exercise training, stem cell therapy, and hyaluronic acid were applied concurrently, they had an additive effect on the number of fibers (F = 174.21, P = 0.001, µ = 0.813) (Figure 4).
5. Discussion
The findings of this study primarily showed that quadriceps muscle fiber count was significantly reduced by the induction of osteoarthritis. There are several possible mechanisms that may explain this reduction: (1) It is reported that expression of MuRF-1 (as one of the major proteins involved in the activation of the muscular atrophy pathway) increases under conditions of knee osteoarthritis induction in quadriceps (19); (2) osteoarthritis intensifies the pain, and due to the decreased motion, the mechanical pressure required to stimulate protein synthesis pathways in the muscle decreases (20, 21). In this regard, De Ceuninck et al. (22) noted that in the conditions of hip and knee articular osteoarthritis, lower limb muscle strength reduces. These changes in muscle protein content, bring about atrophy and subsequently cause muscle strength to similarize to non-training and inactive conditions (23); (3) another possible mechanism for osteoarthritis-affected muscle mass decrease is inflammation (24, 25). In osteoarthritis, serum concentrations of inflammatory markers increase, which is correlated with decreased muscle mass, physical function, and muscle strength (26, 27).
Aerobic exercise training, stem cell therapy, and hyaluronic acid treatment may have a positive effect on the muscle affected by osteoarthritis. Aerobic training may reduce the process of muscle destruction in osteoarthritis patients through reducing inflammation, an issue that needs further researches. There are several possible mechanisms regarding the effect of stem cell therapy. There is evidence that cell therapy can heal damaged muscles (28). It has been reported that in rats with muscle destruction, stem cell infusion induced new myofibrils and stimulate satellite cell activity (29). On the other hand, stem cells can modulate macrophage activity and thus inhibit inflammatory processes that lead to muscle atrophy (30). Regarding hyaluronic acid, it has been reported that the use of hyaluronic acid can reduce pain in osteoarthritic conditions and increase joint function (31). Accordingly, reducing pain increases the motor ability and subsequently increases one’s ability to perform physical activity, which can reduce muscle atrophy processes and maintain muscle mass and subsequently, muscle strength.
5.1. Conclusions
The present study showed that the concurrent use of aerobic exercise training, stem cell, and hyaluronic acid treatments had more effect than each intervention alone on enhancing the muscle fiber count in the rat model of knee osteoarthritis. Therefore, it is recommended that in the induced osteoarthritis model, concurrent use of these three interventions could be effective in reducing muscle damage as one of the major complications of osteoarthritis. However, further studies are needed, particularly in the human model, to better understand the effects of these interventions.